
LL ORG CHEM
6th Edition
ISBN: 9781264840083
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 63P
Draw in all the carbon and hydrogen atoms in each molecule.
a. 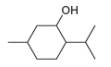 c.
c. 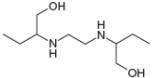
methanol ethambutol
(isolated from peppermint oil) (drug used to treat tuberculosis)
b. 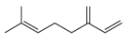 d.
d. 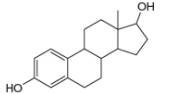
myrcene estradiol
(isolated from bayberry) (a female sex harmone)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a)
NHBoc
⚫OBn
HO.
H3C
CO2CH3
-OBn
H3C
H3C.
H3C.
NHBOC
CI
CO2CH3
Draw structures of the following compounds and identify their role:
mCPBA
(MCPBA)
DMS
Py
9-BBN
LAH
Sia₂BH
TsCI
PCC
t-BuOK
LDA
MeLi
n-BuLi
DMSO
DMF
Sodium Borohydride
Lithium DiisopropylAmide
2
Using Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode.
DATA: Electrode potentials E° = 0,854 V y E 0,788 V
Hg2+/Hg
2+
Hg2/Hg
Chapter 1 Solutions
LL ORG CHEM
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forwardidentify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: ? or CI CI 1) NaNHz (excess) 2) H₂O OA OB O C OD OE OF H₂SO₂, H₂O Hq50. 1) n-BuLi 2) Br 1) 9-BBN 2) H₂O₂, NaOH A B H H متته D E H H H H C H H F H H H₂ Lindlar's catalyst Na NHarrow_forward
- Identify the product (or multiple products) for each of the following reactions: O A OB Oc OD OE OF CI CI 1) NaNH2 (excess) 2) H₂O H₂ H₂SO2, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) Br 1)9-BBN 2) H₂O₂, NaOH ? Na, NH3 C H A H H مننه مننه منن مننه H F H H E مند H D H Harrow_forwardFor the following compound: HO H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: + H-O: H-OH₂ H Mechanism B: H-Ö: HO-H H-OO -H H HH H H HH H-O: H-OO H-OO -H H e -H : OH Θ Mechanism C: Θ A : OH H-O: H H H-O-H 0. Mechanism D: e.. : OH :0 H H-O-H H-O: H-OO :O H -H H H сём H 0 :0 + H Θ H H H-arrow_forwardFor the following compound: H OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Θ :OH O O-H HO 0: Mechanism B: :O-H e.. Θ :OH Mechanism C: H HO-H :0: Θ 0: H H e.. : OH 0: "Θ HH O. :OH :OH O-H O-H Mechanism D: :OH H-OH₂ :OH HO-H 0: © O-H H HH 0: HHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY