yur as marking consists protein mat calowing Wi/ a 125 KD heteromer c de are of each subunt masses: 25 kp, 40KD, and спе disutide band farmed betweeth cys residues in шала maechar CU. KD The protein. has 25 and 60 Kb be The absented. molectar subunits. What. masses (mass) in by If the protein was analyzed under. (a) gel (iltration native condition stography options: 25, 40, 60, 65, 85, 125, 'not applicable The (6) sps- page. (1) gel of in Aitration 8. M. presence of mercaptorethanal anranctography 。ahranctography in the presence
yur as marking consists protein mat calowing Wi/ a 125 KD heteromer c de are of each subunt masses: 25 kp, 40KD, and спе disutide band farmed betweeth cys residues in шала maechar CU. KD The protein. has 25 and 60 Kb be The absented. molectar subunits. What. masses (mass) in by If the protein was analyzed under. (a) gel (iltration native condition stography options: 25, 40, 60, 65, 85, 125, 'not applicable The (6) sps- page. (1) gel of in Aitration 8. M. presence of mercaptorethanal anranctography 。ahranctography in the presence
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
#9

Transcribed Image Text:**Title: Analyzing a Heteromeric Protein through Various Biochemical Methods**
**Introduction:**
When studying proteins, understanding their structure and subunit composition is crucial. This guide explores how to analyze a heteromeric protein using different techniques and conditions.
**Protein Structure:**
You are examining a 125 kDa heteromeric protein composed of one of each subunit with the following molecular masses: 25 kDa, 40 kDa, and 60 kDa. The protein has disulfide bonds formed between cysteine residues in the 25 kDa and 60 kDa subunits.
**Objective:**
Determine the observed molecular masses (in kDa) of the protein under various analytic conditions:
**Methods:**
**(a) Gel Filtration Chromatography under Native Conditions**
Options:
- 25 kDa
- 40 kDa
- 60 kDa
- 65 kDa
- 125 kDa
- Not applicable
**(b) SDS-PAGE in the Presence of Mercaptoethanol**
This method involves using SDS-PAGE, where mercaptoethanol reduces disulfide bonds, potentially altering the protein’s migration pattern.
**(c) Gel Filtration Chromatography in the Presence of 8 M Urea**
Urea can disrupt non-covalent interactions, affecting protein structure and separation during chromatography.
**Conclusion:**
Each method provides insights into the protein's composition and interactions. By analyzing the results from these techniques, we can infer the protein's subunit structure and bonding.
**Note:**
Ensure proper laboratory techniques and conditions are followed while conducting these experiments for accurate results.
Expert Solution
Step 1: Structure of the given heterotrimeric protein
The structure of the 125 kDa heterotrimeric protein given in question is depicted below.
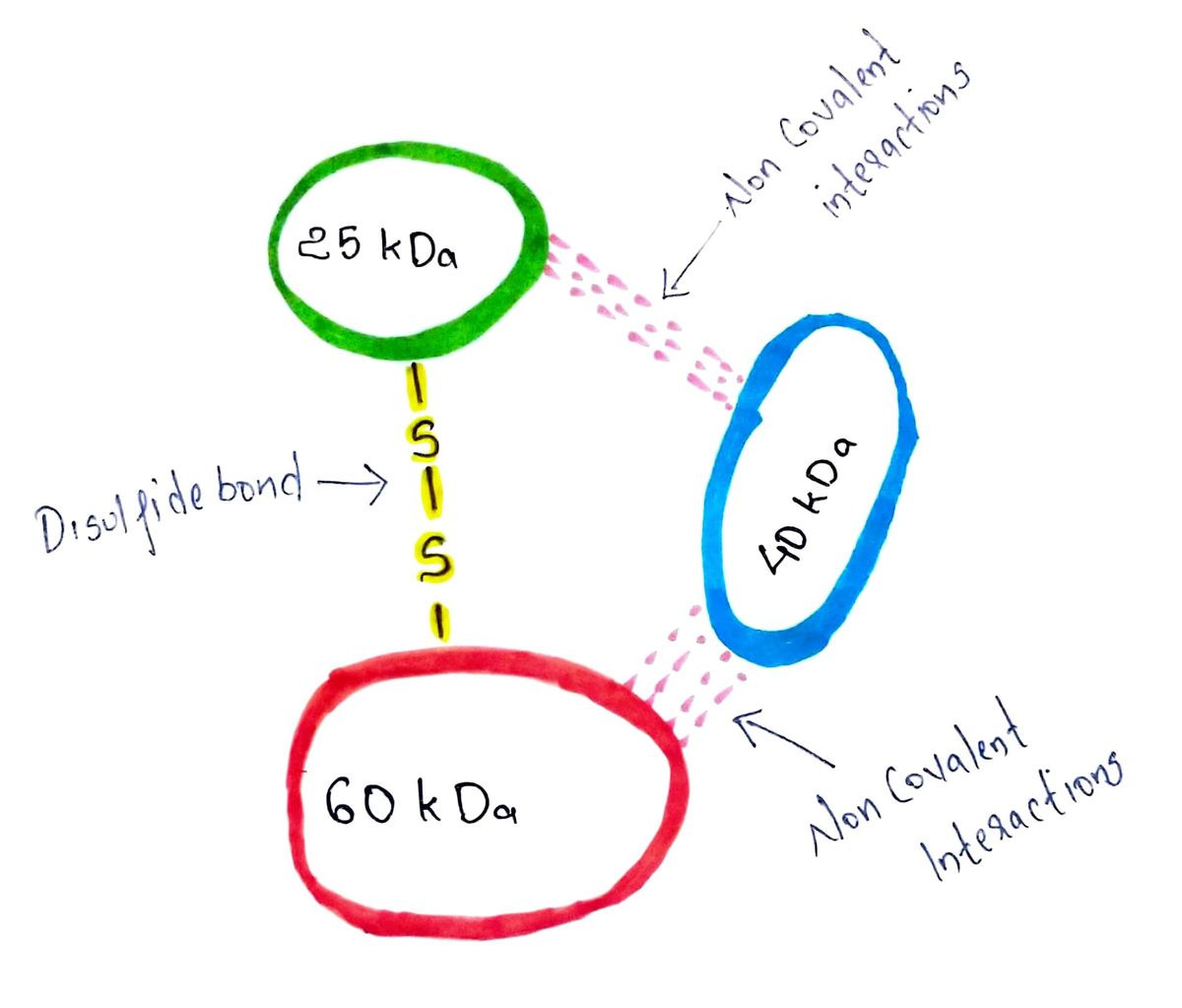
It is composed of 3 subunits; a 25 kDa, a 40 kDa and a 60 kDa subunit.
The 25 kDa and 60 kDa subunits are connected to each other via a disulfide bridge (-S-S-). Connections with 40 kDa subunit are not mentioned in question and hence it must be having non-covalent interactions with at least one of the 25 kDa or 60 kDa subunits, which keeps the 40 kDa subunit connected to the other two subunits.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON