X-RAY RADIATION Constants Units of Energy h = 6.626 · 10-34 J.sec Planck constant e = 1.6· 10-19 C charge of electron c = 3. 10 m/sec speed of light m = 9.11· 10-31 kg mass of electron 1 eV = 1,602-10- J 1 kev = 1,602-10- J 1. Relationship between X-ray frequency and wavelength: c = fA here A is wavelength (m), f is frequency (Hz), c is speed of light (m/sec). K- cathode A - anode Х-гауs electrons 2. Energy of X-rays photon: E = hfmax = Атin Amin is minimum wavelength of X-ray radiation (m), fmax is maximum frequency of X-ray radiation (Hz), h is Planck constant (J-sec), is speed of light propagation in vacuum (m/sec). 3. In the X-ray tube, firstly, the energy of electric field is transformed into the kinetic energy of electron motion: ту? eU, = and then the kinetic energy of electron motion is transferred into energy of X-ray photons: ту? 7 = h fmax is maximum frequency of X-ray radiation (Hz), h is Planck constant (J-sec), c is speed of fmax light propagation in vacuum (m/sec), e is charge of electron (C), m mass of electron (kg), Ug is potential difference or voltage between cathode and anode (V), v is speed of electrons motion (m/sec). 4. Relationship between wavelenth of X-ray radiation and Voltage on the tube: Amin(nm) = v.(kV) 1.23 U, is potential difference or voltage between cathode and anode (V), Amin is minimum wavelength of X-ray radiation (m). 5. Linear attenuation coefficient (1/m): µ = k•p•i² •Z³, k = 10-9 is coefficient of proportionality (W/V²A), p is density of the material (kg/m³), Z is atomic number of the material, i is wavelength of X-ray radiation (m). 6. Mass attenuation coefficient (m/kg): Hm = = ka³23 µ is linear attenuation coefficient, k = 10-9 is coefficient of proportionality (W/VA), p is density of the material (kg/m'), Z is atomic number of the material, i is wavelength of X-ray radiation (m). 7. Attenuation of X-ray radiation intensity: | = Le-ud here I, is the initial intensity of X-rays (W/m?); / is the intensity of X-rays after passing a material of thickness d (W/m?), d is thickness of the adsorbing layer (m), u is linear attenuation coefficient (1/m). 8. Half value layer: In(2) 0,693 HVL = u is linear attenuation coefficient (1/m). 7. (a) What is the energy of an X-ray photons, if it corresponds to a wavelength of 0.005 nm? (b) Find corresponding frequency of X-ray radiation.
X-RAY RADIATION Constants Units of Energy h = 6.626 · 10-34 J.sec Planck constant e = 1.6· 10-19 C charge of electron c = 3. 10 m/sec speed of light m = 9.11· 10-31 kg mass of electron 1 eV = 1,602-10- J 1 kev = 1,602-10- J 1. Relationship between X-ray frequency and wavelength: c = fA here A is wavelength (m), f is frequency (Hz), c is speed of light (m/sec). K- cathode A - anode Х-гауs electrons 2. Energy of X-rays photon: E = hfmax = Атin Amin is minimum wavelength of X-ray radiation (m), fmax is maximum frequency of X-ray radiation (Hz), h is Planck constant (J-sec), is speed of light propagation in vacuum (m/sec). 3. In the X-ray tube, firstly, the energy of electric field is transformed into the kinetic energy of electron motion: ту? eU, = and then the kinetic energy of electron motion is transferred into energy of X-ray photons: ту? 7 = h fmax is maximum frequency of X-ray radiation (Hz), h is Planck constant (J-sec), c is speed of fmax light propagation in vacuum (m/sec), e is charge of electron (C), m mass of electron (kg), Ug is potential difference or voltage between cathode and anode (V), v is speed of electrons motion (m/sec). 4. Relationship between wavelenth of X-ray radiation and Voltage on the tube: Amin(nm) = v.(kV) 1.23 U, is potential difference or voltage between cathode and anode (V), Amin is minimum wavelength of X-ray radiation (m). 5. Linear attenuation coefficient (1/m): µ = k•p•i² •Z³, k = 10-9 is coefficient of proportionality (W/V²A), p is density of the material (kg/m³), Z is atomic number of the material, i is wavelength of X-ray radiation (m). 6. Mass attenuation coefficient (m/kg): Hm = = ka³23 µ is linear attenuation coefficient, k = 10-9 is coefficient of proportionality (W/VA), p is density of the material (kg/m'), Z is atomic number of the material, i is wavelength of X-ray radiation (m). 7. Attenuation of X-ray radiation intensity: | = Le-ud here I, is the initial intensity of X-rays (W/m?); / is the intensity of X-rays after passing a material of thickness d (W/m?), d is thickness of the adsorbing layer (m), u is linear attenuation coefficient (1/m). 8. Half value layer: In(2) 0,693 HVL = u is linear attenuation coefficient (1/m). 7. (a) What is the energy of an X-ray photons, if it corresponds to a wavelength of 0.005 nm? (b) Find corresponding frequency of X-ray radiation.
College Physics
11th Edition
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter1: Units, Trigonometry. And Vectors
Section: Chapter Questions
Problem 1CQ: Estimate the order of magnitude of the length, in meters, of each of the following; (a) a mouse, (b)...
Related questions
Question

Transcribed Image Text:X-RAY RADIATION
Constants
Units of Energy
h = 6.626 · 10-34 J.sec Planck constant
e = 1.6· 10-19 C charge of electron
c = 3. 10 m/sec speed of light
m = 9.11· 10-31 kg mass of electron
1 eV = 1,602-10- J
1 kev = 1,602-10- J
1. Relationship between X-ray frequency and
wavelength:
c = fA
here A is wavelength (m), f is frequency (Hz), c
is speed of light (m/sec).
K- cathode
A - anode
Х-гауs
electrons
2. Energy of X-rays photon:
E = hfmax =
Атin
Amin is minimum wavelength of X-ray radiation (m), fmax is maximum frequency of X-ray radiation
(Hz), h is Planck constant (J-sec), is speed of light propagation in vacuum (m/sec).
3. In the X-ray tube, firstly, the energy of electric field is transformed into the kinetic energy of
electron motion:
ту?
eU, =
and then the kinetic energy of electron motion is transferred into energy of X-ray photons:
ту?
7 = h fmax
is maximum frequency of X-ray radiation (Hz), h is Planck constant (J-sec), c is speed of
fmax
light propagation in vacuum (m/sec), e is charge of electron (C), m mass of electron (kg), Ug is
potential difference or voltage between cathode and anode (V), v is speed of electrons motion
(m/sec).
4. Relationship between wavelenth of X-ray radiation and Voltage on the tube:
Amin(nm) = v.(kV)
1.23
U, is potential difference or voltage between cathode and anode (V), Amin is minimum wavelength
of X-ray radiation (m).
5. Linear attenuation coefficient (1/m):
µ = k•p•i² •Z³,
k = 10-9 is coefficient of proportionality (W/V²A), p is density of the material (kg/m³), Z is atomic
number of the material, i is wavelength of X-ray radiation (m).
6. Mass attenuation coefficient (m/kg):
Hm =
= ka³23
µ is linear attenuation coefficient, k = 10-9 is coefficient of proportionality (W/VA), p is density
of the material (kg/m'), Z is atomic number of the material, i is wavelength of X-ray radiation (m).
7. Attenuation of X-ray radiation intensity:
| = Le-ud
here I, is the initial intensity of X-rays (W/m?); / is the intensity of X-rays after passing a material of
thickness d (W/m?), d is thickness of the adsorbing layer (m), u is linear attenuation coefficient
(1/m).
8. Half value layer:
In(2)
0,693
HVL =
u is linear attenuation coefficient (1/m).

Transcribed Image Text:7. (a) What is the energy of an X-ray photons, if it corresponds to a wavelength of 0.005 nm?
(b) Find corresponding frequency of X-ray radiation.
Expert Solution
Step 1
Given,
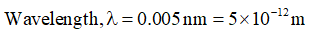
Step 2
(a) Energy of wave is given by, 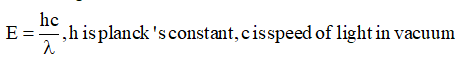
Substituting values,
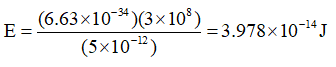
Ans. Energy of X-ray is 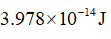
Step by step
Solved in 3 steps with 7 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics (14th Edition)
Physics
ISBN:
9780133969290
Author:
Hugh D. Young, Roger A. Freedman
Publisher:
PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:
9781107189638
Author:
Griffiths, David J., Schroeter, Darrell F.
Publisher:
Cambridge University Press

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics (14th Edition)
Physics
ISBN:
9780133969290
Author:
Hugh D. Young, Roger A. Freedman
Publisher:
PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:
9781107189638
Author:
Griffiths, David J., Schroeter, Darrell F.
Publisher:
Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:
9780321820464
Author:
Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:
Addison-Wesley

College Physics: A Strategic Approach (4th Editio…
Physics
ISBN:
9780134609034
Author:
Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:
PEARSON