Write the Nernst equation2 for the reaction Cu2+ (aq) + Zn (s) ® Zn2+ (aq) + Cu (s)
Let activity of Cu2+ equal to 1 and change the activity of Zn2+ such that Q is in the range - 0.001 to +0.001. Select different values of Q and for each calculate Ecell. Plot ?cell−?cellө vs ln Q. What is the effect of Q (that is the activity) on ?cell−?cellө ? Is the effect the same at higher and lower values of Q? what is the largest value of the absolute difference between cell potential and standard cell potential, |?cell−?cellө|? What do you expect the graph will look like for different number of electron transferred (n )? And why?
Nernst equation is used to determine the emf of the cell.
Nernst equation:
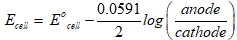
Nernst equation for zinc and copper cell.
The balanced chemical equation:
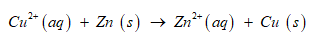
Cell representation:
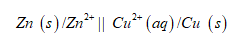
Oxidation of zinc take place and reduction of copper is taking place.
Zinc oxidize to Zn2+ and Cu2+ reduced to Cu.
Nernst equation is:
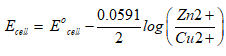
Step by step
Solved in 3 steps with 6 images









