Write if the reactions are spontaneous or not. Br- | Br2 || H+| H2 Net ionic eq: Anode: E°= Cathode: E°= __________________________________________________________ Ionic eq: E°=
Answer the following. Write if the reactions are spontaneous or not.
Br- | Br2 || H+| H2
Net ionic eq:
Anode: E°=
Cathode: E°=
__________________________________________________________
Ionic eq: E°=
The left side of the cell representation is the anode and the right side of the cell representation is the cathode. At the anode, the loss of electrons take place, that is the oxidation and at the cathode, the gain of electrons take place, that is the reduction.
The given cell representation is,
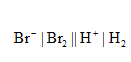
The reaction at the anode is,

The reaction at the cathode is,

The ionic equation is,
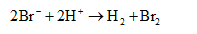
The electrode potential of the cell can be calculated as shown below.
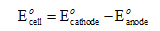
Substitute the values in the above equation to calculate the electrode potential of the cell.
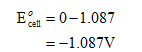
Step by step
Solved in 2 steps with 8 images









