Write a balanced standard formation reaction and a balanced standard combustion reaction for each of the following compounds, and For each of the compounds in Q-9, calculate the standard enthalpy of combustion using enthalpies of formation from the table of thermodynamic properties a) CH4 (methane) b) C6H12O6 (s) [ glucose ]
- Write a balanced standard formation reaction and a balanced standard combustion reaction for each of the following compounds, and For each of the compounds in Q-9, calculate the standard enthalpy of combustion using enthalpies of formation from the table of
thermodynamic properties - a) CH4 (methane)
- b) C6H12O6 (s) [ glucose ]
Given compounds are methane (CH4) and glucose (C6H12O6).
The values of enthalpies of formation for different compounds are taken from the table of thermodynamic properties.
The balanced standard formation reaction is written as follows:
- Methane (CH4)
When 1 mole of carbon reacts with 2 moles of hydrogen, 1 mole of methane is formed.
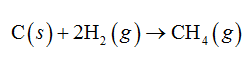
b. Glucose (C6H12O6)
When 6 moles of carbon reacts with 6 moles of water, 1 mole of glucose is formed.
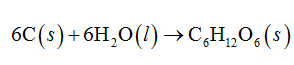
The balanced standard combustion reaction is written as follows:
- Methane (CH4)
A combustion reaction is a reaction in which a compound is burnt in the presence of oxygen.
In the combustion of methane, 1 mole of methane is burnt with the help of 2 moles of oxygen to form 1 mole of carbon dioxide and 2 moles of water vapor.
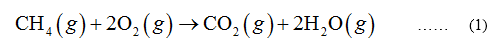
b. Glucose (C6H12O6)
In the combustion of glucose, 1 mole of glucose is burnt with the help of 6 moles of oxygen to form 6 moles of carbon dioxide and 6 moles of water.
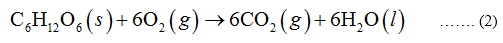
Step by step
Solved in 5 steps with 8 images









