Why does CsCl present a body-centered cubic lattice?
Crystalline solids are mainly characterized by regular and repeating pattern of its constituent particles. If this three dimensional arrangement of the constituent particles in a crystal is to be represented diagrammatically, each particle will be depicted as a point, giving rise to an arrangement known as crystal lattice.
A part of the crystal lattice is shown in the diagram below:
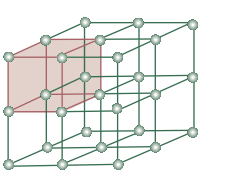
A unit cell refers to the smallest portion of the crystal lattice which when repeated in different leads to the formation of entire crystal lattice.They are mainly divided into two categories:
- Primitive unit cell- If the constituent particles are present only at the corners of the unit cell ,then such type of cell is known as primitive unit cell.
Centered unit cells- If in the unit cell one or more constituent particles at positions other than corners than ,such type of unit cells are referred to as centered unit cells. They are mainly of three types:-
- Body centered- These are the type of unit cells which contain one atom or particle at its body-center besides the ones that are preset at the corners.
- Face centered- These are the type of unit cells which contain one atom or particle at the center of the each face, besides the ones which are present at the corners.
- End-centered- In these type of unit cells ,one atom or particle is present at the center of any of the two opposite faces besides the ones which are present at the corners of the unit cell.
Step by step
Solved in 3 steps with 2 images









