Which of the following scientists first populated the sharp lines in the emission spectra of elements were caused by electrons going from high energy levels to low energy levels?
Which of the following scientists first populated the sharp lines in the
The emission of radiation given by an energized hydrogen atom to the electron falling from a higher-energy orbit to a lower orbit give a quantum of energy in the form of light Based on electrostatic interaction and law of motion, Bohr derived the following equation.
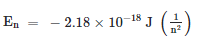
where n gets an integer values such as n = 1, 2, 3 and so on. This is the energy of electron in nth orbital.
The electrons are excited thermally when the light is used by an object. As a result, an emission spectrum comes. Line spectra consist of light only at specific, discrete wavelengths. In emission, the electron returns to a lower energy state from nf (the i and f subscripts denote the initial and final energy states). In most cases, the lower energy state corresponds to the ground state but it may be any energy state which is lower than the initial excited state. The difference in the energies between the initial and final states is,
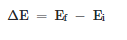
This transition results in the photon's emission with frequency v and energy hv. The following equation is resulted.
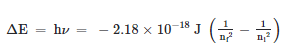
When ni > nf a photon is emitted. The term in parentheses is positive, making ΔE negative. As a result, energy is lost to the surroundings. When n; < nf, a photon is absorbed. The term in parentheses is negative, so ΔE is positive. As a result, energy is absorbed from the surroundings.
Bohr's theory of the hydrogen atom tells an atom as a small, positively charged nucleus with electrons which travels around the nucleus in circular orbits. The appearance of an emission spectrum is explained because the "orbits" available for the electrons to occupy in their excited states are quantized. The resulting transition emits a specific wavelength of light because the energy of the "orbit" is quantized.
Step by step
Solved in 2 steps with 3 images









