Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
i neep help please

Transcribed Image Text:**Question:**
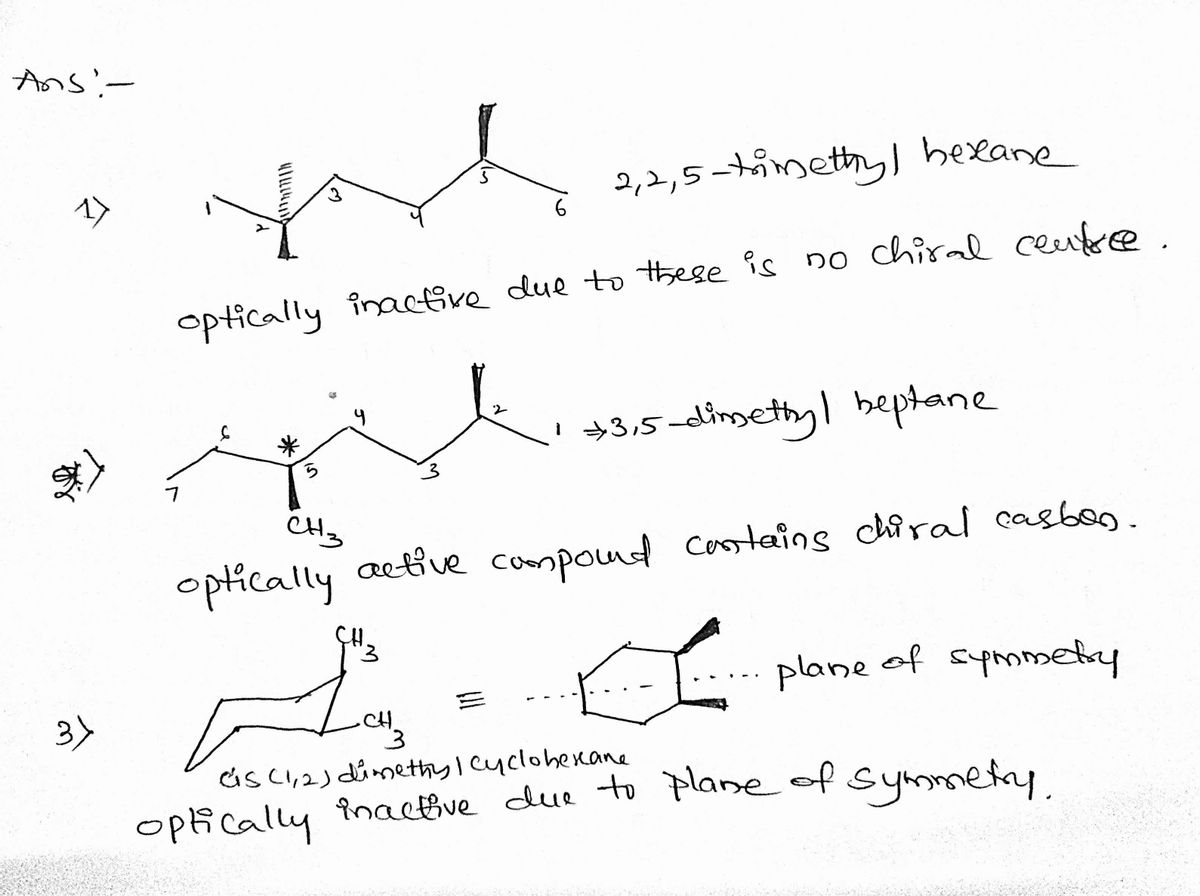
Which of the following molecules is optically active?
**Options:**
1. A molecule with a chiral carbon bound to three different groups and one lone hydrogen (CH₃, wedge and dash bonds visible).
2. A longer carbon chain with one chiral center denoted by a wedge and dash, including a CH₃ group.
3. A cyclohexane ring with two methyl (CH₃) groups attached at different positions.
**Explanation of Diagrams:**
- **First Molecule:**
- Features a single chiral center, indicated by a wedge and a dash for bonds, suggesting potential optical activity.
- **Second Molecule:**
- Contains a chiral center marked by a wedge and dash at different points along the carbon chain. This chiral center contributes to its optical activity.
- **Third Molecule:**
- Shows a cyclic structure with two substituents. The symmetry of the cyclohexane means there's no chiral center.
**Note:** Optical activity in compounds arises when there is a non-superimposable mirror image, often due to the presence of a chiral center (asymmetric carbon atom).

Transcribed Image Text:### Chemical Structure Diagrams
1. **Diagram 1:**
- **Description:** This structure represents a linear alkane in a zig-zag formation, common in organic chemistry representations. It includes two methyl groups (CH₃) attached at opposite ends.
2. **Diagram 2:**
- **Description:** This structure shows a cyclobutane ring with two substituents. One methyl group (CH₃) is attached with a wedge bond indicating it is coming out of the plane, and another methyl group is represented with a dashed bond indicating it is going into the plane. This denotes stereochemistry, specifying the 3D orientation of the groups.
3. **Diagram 3:**
- **Description:** This structure depicts a cyclopentane ring. Two methyl groups are attached on opposite carbon atoms, one with a wedge bond (out of the plane) and the other with a dashed bond (into the plane), also indicating stereochemistry.
Each diagram includes a selection checkbox, suggesting an interactive component, potentially for quizzes or assessments. The structures are shaded differently, possibly for educational emphasis.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY