Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:Which atomic spectrometer has the highest atomization temperature? What is the
advantage of having such a higher atomization temperature?
Expert Solution
Step 1
- Inductively coupled plasma atomic emission spectroscopy (ICP-AES), also referred to as inductively coupled plasma optical emission spectroscopy (ICP-OES) has the highest atomization temperature nearly 8000 K.
- Many different plasma sources exist, but by far the most common is the inductively coupled plasma
(ICP). The ICP is generated by coupling the energy from a radiofrequency generator into a suitable gas
via a magnetic field which is induced through a two- or three-turn, water-cooled copper coil. The
radiofrequency energy is normally supplied at a frequency of 27.12 MHz, delivering forward power at
between 500 and 2000 W. Two gas flows, usually argon, flow in a tangential manner through the outer
tubes of a concentric, three-tube quartz torch which is placed axially in the copper coil .
Because the outer and intermediate gases flow tangentially (i.e. they swirl around as they pass
through the torch), the plasma is continually revolving and has a 'weak spot' at the centre of its base,
through which the inner gas flow, containing the sample, can be introduced. When the gas is seeded
with electrons, usually by means of a spark, the electrons accelerate in the magnetic field and reach
energies sufficient to ionize gaseous atoms in the field. Subsequent collisions with other gaseous atoms
causes further ionization and so on, so that the plasma becomes self-sustaining. This occurs almost
instantaneously. The magnetic field causes the ions and electrons to flow in the horizontal plane of the
coil, thereby heating the neutral argon by collisional energy exchange, and a hot fireball is produced.
The hottest part of the ICP has a temperature between 8000.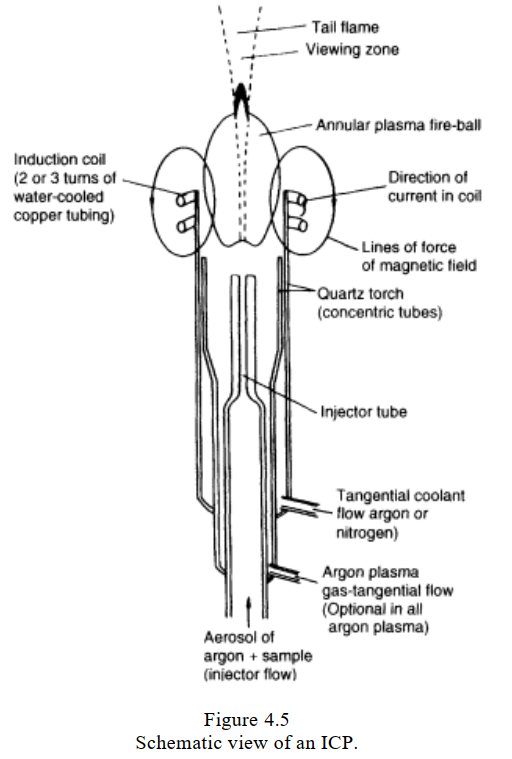
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY