Which alcohol is formed from the acid-catalyzed hydration of the following alkene? H CH3 H,0 H2SO4 CH,CH, CH3 но-с-с-н CH,CH, H H CH3 CH, H-C-C-OH CH,CH, HO CH, c-C CH,CH, но-с-с-SH H CH,CH, 1 Select one: О а. 1 О b. 2 О с. 3 O d. 4
Which alcohol is formed from the acid-catalyzed hydration of the following alkene? H CH3 H,0 H2SO4 CH,CH, CH3 но-с-с-н CH,CH, H H CH3 CH, H-C-C-OH CH,CH, HO CH, c-C CH,CH, но-с-с-SH H CH,CH, 1 Select one: О а. 1 О b. 2 О с. 3 O d. 4
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Question:** Which alcohol is formed from the acid-catalyzed hydration of the following alkene?
**Reaction Scheme:**
\[ \text{Alkene:} \quad \]
\[ \text{H}_3\text{C} \text{C} = \text{C} \text{H}_ \]
\[ \text{H} \quad \text{H CH}_2\text{CH}_3 \]
**Reagents:**
- \(\text{H}_2\text{O} + \text{H}_2\text{SO}_4\)
**Products:**
1. \(\text{HO}-\text{C}-\text{C}-\text{CH}_3\)
\[ \text{H CH}_2\text{CH}_3 \]
\[\text{H}\]
2. \(\text{HO}-\text{C}-\text{C}-\text{CH}_2\text{CH}_3\)
\[ \text{H CH}_3 \]
\[\text{H}\]
3. \(\text{H}-\text{C}-\text{C}-\text{OH}\)
\[ \text{H CH}_3 \]
\[\text{CH}_2\text{CH}_3\]
4. \(\text{HO} \text{C} = \text{C} \text{H}_3\)
\[\text{H}\]
\[\text{CH}_2\text{CH}_3\]
**Question:**
Select one:
- a. 1
- b. 2
- c. 3
- d. 4
**Instructions for Students:**
Review the structure of each alcohol product carefully. Consider the mechanism of acid-catalyzed hydration of alkenes to predict which product forms. Decide which number corresponds to the correct product and select the appropriate option.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3b05a37d-01cc-41b5-b183-5c50b16f16a3%2F459294a6-08c0-4ab2-91a2-a945e6b5774d%2Fdcfkde_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Question:** Which alcohol is formed from the acid-catalyzed hydration of the following alkene?
**Reaction Scheme:**
\[ \text{Alkene:} \quad \]
\[ \text{H}_3\text{C} \text{C} = \text{C} \text{H}_ \]
\[ \text{H} \quad \text{H CH}_2\text{CH}_3 \]
**Reagents:**
- \(\text{H}_2\text{O} + \text{H}_2\text{SO}_4\)
**Products:**
1. \(\text{HO}-\text{C}-\text{C}-\text{CH}_3\)
\[ \text{H CH}_2\text{CH}_3 \]
\[\text{H}\]
2. \(\text{HO}-\text{C}-\text{C}-\text{CH}_2\text{CH}_3\)
\[ \text{H CH}_3 \]
\[\text{H}\]
3. \(\text{H}-\text{C}-\text{C}-\text{OH}\)
\[ \text{H CH}_3 \]
\[\text{CH}_2\text{CH}_3\]
4. \(\text{HO} \text{C} = \text{C} \text{H}_3\)
\[\text{H}\]
\[\text{CH}_2\text{CH}_3\]
**Question:**
Select one:
- a. 1
- b. 2
- c. 3
- d. 4
**Instructions for Students:**
Review the structure of each alcohol product carefully. Consider the mechanism of acid-catalyzed hydration of alkenes to predict which product forms. Decide which number corresponds to the correct product and select the appropriate option.
Expert Solution
Step 1
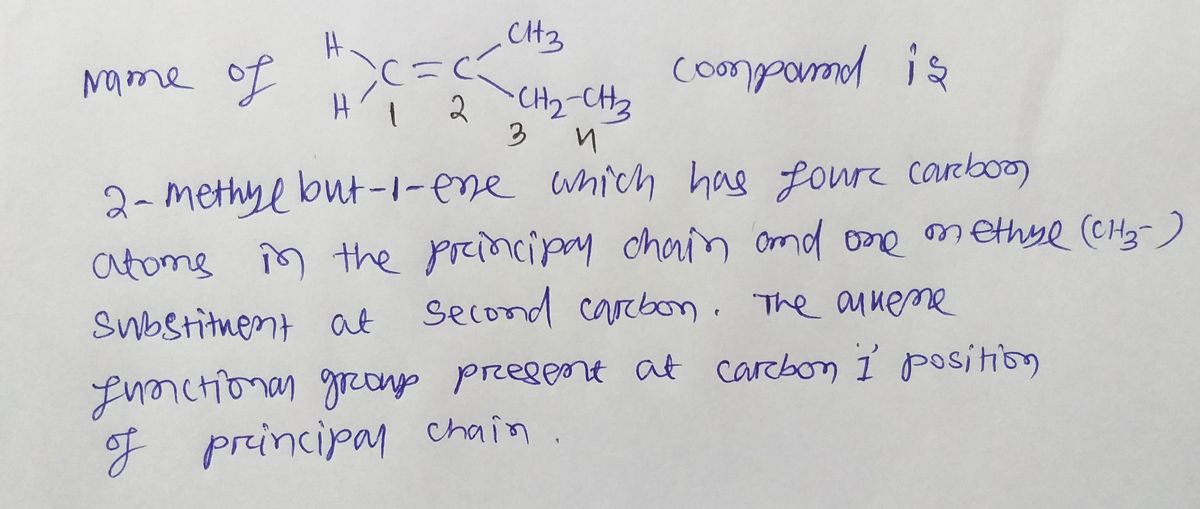
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY