What is the significance of pH value? What makes something basic vs acidic?
Autoionization of water is a process through which water molecules generate hydrogen ions and hydroxide ions by dissociation reaction in equilibrium.
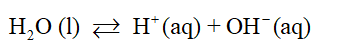
pH is a measure to though which we can know the acidic or basic nature of a solution. pH scale ranges from 0-14 where 0 represents most acidic and 14 represents the most basic or alkaline solution. The mid-value of the pH scale i.e., 7 is used to represent neutral solutions. pH scale helps to rank the acids and bases.
significance of pH value
maintenance of pH is very important for bodily functions such as digestion. Our stomach has HCl to maintain acidic conditions in the stomach to activate pepsin for protein breakdown whereas our intestine has slightly alkaline pH to activate pancreatic enzymes to carry out catalytic conditions. Similarly, our blood is slightly alkaline, and if pH of the blood changes it leads to certain ailments.
In agricultural activities, soil pH is an important factor for plant growth. Not only for plant growth but for the microbial and fungal growth in the soil as well. In aquatic environments fluctuations in pH level hinder the growth and reproduction of aquatic organisms.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images









