Reactive Intermediates
In chemistry, reactive intermediates are termed as short-lived, highly reactive atoms with high energy. They rapidly transform into stable particles during a chemical reaction. In specific cases, by means of matrix isolation and at low-temperature reactive intermediates can be isolated.
Hydride Shift
A hydride shift is a rearrangement of a hydrogen atom in a carbocation that occurs to make the molecule more stable. In organic chemistry, rearrangement of the carbocation is very easily seen. This rearrangement can be because of the movement of a carbocation to attain stability in the compound. Such structural reorganization movement is called a shift within molecules. After the shifting of carbocation over the different carbon then they form structural isomers of the previous existing molecule.
Vinylic Carbocation
A carbocation where the positive charge is on the alkene carbon is known as the vinyl carbocation or vinyl cation. The empirical formula for vinyl cation is C2H3+. In the vinyl carbocation, the positive charge is on the carbon atom with the double bond therefore it is sp hybridized. It is known to be a part of various reactions, for example, electrophilic addition of alkynes and solvolysis as well. It plays the role of a reactive intermediate in these reactions.
Cycloheptatrienyl Cation
It is an aromatic carbocation having a general formula, [C7 H7]+. It is also known as the aromatic tropylium ion. Its name is derived from the molecule tropine, which is a seven membered carbon atom ring. Cycloheptatriene or tropylidene was first synthesized from tropine.
Stability of Vinyl Carbocation
Carbocations are positively charged carbon atoms. It is also known as a carbonium ion.
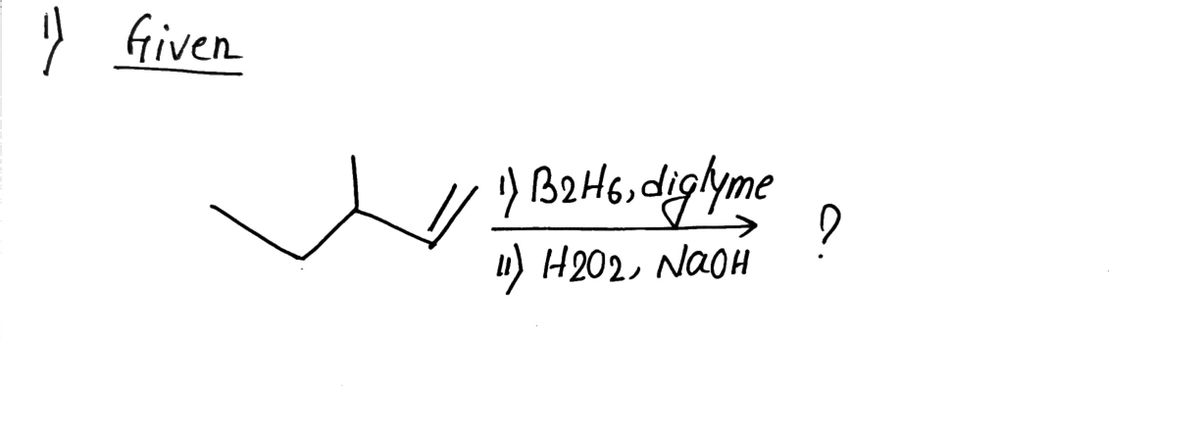
Can you answer both? Thank you!
![**Question:**
What is the major product of the following reaction?
**Reaction:**
Reactant: An alkene with the formula shown in the image.
Reagents:
1) \( \text{B}_2\text{H}_6, \text{diglyme} \)
2) \( \text{H}_2\text{O}_2, \text{NaOH} \)
**Options:**
- **A)**
\[
\begin{array}{c}
\text{A structure with the OH group on the terminal carbon.}
\end{array}
\]
- **B)**
\[
\begin{array}{c}
\text{A structure with the OH group on the middle carbon.}
\end{array}
\]
- **C)**
\[
\begin{array}{c}
\text{A structure with the OH group on the carbon next to the branch.}
\end{array}
\]
- **D)**
\[
\begin{array}{c}
\text{A structure with OH groups on adjacent carbons.}
\end{array}
\]
**Explanation:**
This reaction involves hydroboration-oxidation, where the boron reagent adds across the double bond, followed by oxidation to replace the boron with a hydroxyl group, typically resulting in anti-Markovnikov addition.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5888bae3-212e-47ed-9890-15c9dff3400f%2F41be90fd-ae77-4daf-b453-84fca7fa6e71%2Fcqjcu32_processed.png&w=3840&q=75)

Step by step
Solved in 3 steps with 3 images









