Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
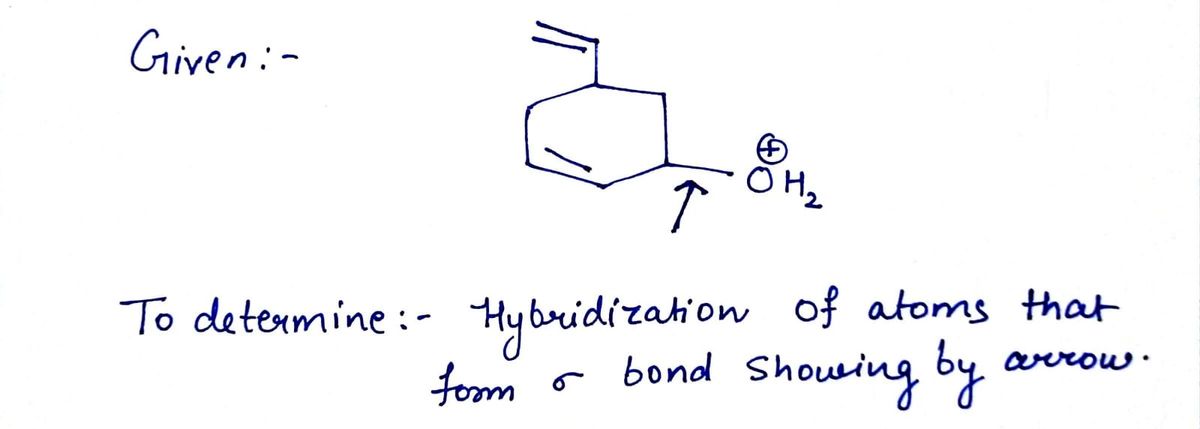
What is the hybridization at the bond being pointed to?

Transcribed Image Text:The image depicts a chemical structure featuring a cyclohexane ring with a substituent. The structure can be broken down as follows:
1. **Cyclohexane Ring**: At the center is a six-membered carbon ring, typical of a cyclohexane. Each vertex of the hexagon represents a carbon atom.
2. **Substituents**:
- At the top of the ring, a linear substituent extends outwards. This implies an additional carbon chain or group connected to the ring.
- Attached to one of the carbon atoms of the cyclohexane is an arrow pointing to the right. Next to this arrow is an “OH2” group, denoting a bonded water molecule segment.
- On the same carbon where the "OH2" is attached, there is a circled positive sign, indicating a positive charge on that carbon atom, suggesting the presence of a carbocation.
This diagram illustrates a carbocation intermediate, often seen in organic reactions such as alkene hydration or rearrangements. The positive charge is essential for understanding the reactivity and mechanism pathways of such compounds.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY