What is the efficiency of a Carnot engine whose isothermal process happen at 30.0 degC and 218 degC?
Q: A Carnot engine's operating temperatures are 200 ∘C∘C and 44 ∘C∘C. The engine's power output is 950…
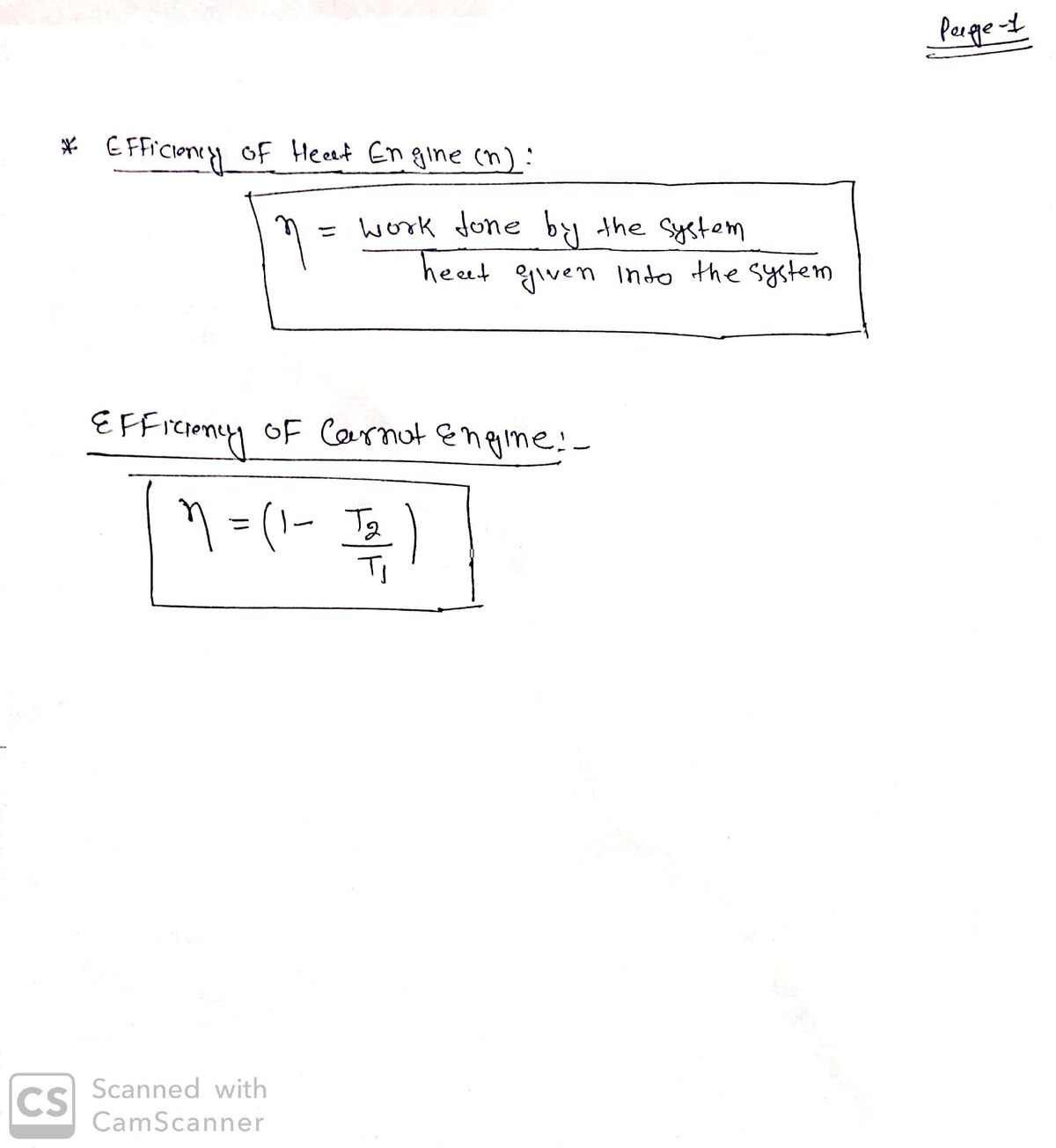
A: The relation for the Carnot's engine is THTc=QHQc where, TH is the temperature of source, Tc is the…
Q: A heat engine has a thermodynamic efficiency of .4. Calculate one cYCLE heat taken up by the engine…
A:
Q: To improve the efficiency of a Carnot engine, you can increase the difference in temperature between…
A:
Q: A Carnot engine operates between the temperatures Th = 100°C and Tc = 23°C. By what factor…
A: Given Data: The temperature of the hot reservoir is, Th=100℃ The temperature of the cold reservoir…
Q: Suppose you want to build a Carnot engine with an efficiency of 80%. If the hot reservoir has a…
A: Given data: A Carnot engine Efficiency (n) = 80% = 0.80 Temperature of hot reservoir (TH) = 1200 K…
Q: In isothermal process, is w = NKTln(v_2/v1) or w = NKTln(v_2 - v1)? w is derived form w =…
A: To derive the equation for the work done in an isothermal process.
Q: Calculate the efficiency of a Carnot engine having a hot reservoir temperature Th and a cold…
A: Given Data, Temperature of Hot reservoir Th=4.00×102 K Temperature of cold reservoir Tc=3.00×102 K
Q: A Carnot engine operates between temperatures of 480 K and 400 K and does 160 J of work in each…
A: Given data *The hot reservoir temperature is T1 = 480 K *The cold reservoir temperature is T2 = 400…
Q: Steam locomotives have an efficiency of 18.2% and operate with a hot steam temperature of 455°C.…
A: Hence the answer in three significant figures is 596.K.
Q: Equivalence of Carnot and Ideal gas Temperature Scale. Consider a Carnot engine working between…
A:
Q: How could you design a Carnot engine with 100% efficiency?
A:
Q: If the cold temperature reservoir of a Carnot engine is held at a constant 324 K , what temperature…
A: Given data: Cold temperature (TC) = 324 K Efficiency (n) = 0.61 Required:. The hot temperature (TH)
Q: in a Carnot engine the temperatures TH and TL have values of 250°C and 150°C. What efficiency, e,…
A: The Carnot efficiency of a heat engine cycle is the maximum theoretical efficiency a heat engine can…
Q: A heat engine operates in a Carnot cycle between 78.0°C and 345°C. It absorbs 20,000 J of energy per…
A: temperature of cold reservoir, Tc =78.0°C = 351 K temperature of hot reservoir, TH = 345°C = 618 K…
Q: a carnot engine has an efficiency of 22%. It operates between two reservoirs differing in…
A: Given: Efficiency of Carnot engine, η=22% Temperature difference between two reservoir, ∆T=75 K To…
Q: A Carnot engine has an efficiency ecarnot= 0.600. The temp. of the hot reservoir is 875 K. part a)…
A: Given:Carnot efficiency, ecarnot = 0.600Temperature of the hot reservoir, TH = 875 KHeat transferred…
Q: A Carnot engine operates between the temperatures Th = 100°C and Tc = 24°C. By what factor does the…
A: Theoretical efficiency of carnot engine : Effc=1-TcTh Given : Th=100°C=100+273=373 KTc=24°…
Q: has No cyclic heat engine reversible engine operating Prove e carnot > e otto with a greater…
A:
Q: certain gasoline engine has an efficiency of 32.0%. What would the hot reservoir temperature (in °C)…
A: Given Temperature of sink = 2100 C = 210+273 = 483 K Efficiency = 32 %
Q: A Carnot engine operates between reservoirs at 630 and 320 K. If the engine absorbs 160 J per cycle…
A: The following question can be solved as follows
Q: What is the total entropy change if a 0.440 efficiency engine takes 3.78E3 J of heat from a 348 degC…
A: To calculate the total entropy change, we can use the equation:ΔS = Q / Twhere ΔS is the total…
Q: n one cycle a heat engine absorbs 460 J from a high-temperature reservoir and expels 310 J to a…
A: Efficiency of the engine ηh=1-QcQh55%ηcarnot=1-QcQh0.551-TlowThigh=1-QcQhTlowThigh=1-10.551-QcQh
Q: A Carnot heat engine operates between tc=252 k and the=466k What is its efficiency?
A:

Step by step
Solved in 2 steps with 2 images

- A particular heat engine has a mechanical power output of 4.20 kW and an efficiency of 26.0%. The engine expels 8.15 103 J of exhaust energy in each cycle. (a) Find the energy taken in during each cycle. KJ (b) Find the time interval for each cycle. sCalculate the net work output of a heat engine following path ABCDA in the figure below, where V1 = 2.6 ✕ 10−3 m3 and V2 = 10.4 ✕ 10−3 m3.A particular heat engine has a mechanical power output of 5.20 kW and an efficiency of 26.0%. The engine expels 7.40 x 103 J of exhaust energy in each cycle. (a) Find the energy taken in during each cycle. kJ (b) Find the time interval for each cycle. S
- The efficiency of all real engines are less than that of the carnot engine. Why?QUESTION 4 In a single cycle, heat engine extracts 8.00 kcal of heat from a hot reservoir and exhausts 5.00 kcal of heat int a cold reservoir. What is the efficiency of this engine? а. 67.5% b. 77.5% C. 57.5% d. 47.5% е. 37.5%