what is the difference b/n spontaneity criterial of simple and non simple systems in terms of gibbs and helmholtz free energies?
Simple system is the single state system having no internal boundaries.
Non simple system has barrier which restrict to transfer energy or matter.
It is the maximum amount of energy is available to a system during process to do some useful work.
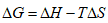
∆G is the change in Gibbs free energy.
∆H is the change in enthalpy.
∆S is the change in entropy.
T is the temperature.
For a spontaneous process, change in Gibbs free energy, defined at constant temperature and pressure, is negative because the energy of the system is decreases as the system moves from higher state of energy to lower state of energy.
The criteria for spontaneity in terms of Gibbs free energy change is:
If Δ G is negative, process is spontaneous
If Δ G is positive, process is non-spontaneous
If Δ G is zero, process is in equilibrium.
Step by step
Solved in 3 steps with 2 images









