Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

**Note:** Understanding the mechanism of electrophilic addition will help determine the correct alkene choice for producing the target alcohol.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F2792385f-8eab-428d-bf5a-ca14f438148e%2F795123c7-77b4-4468-bf66-6cfc0b7b4f87%2Feraqdmg_processed.png&w=3840&q=75)
Transcribed Image Text:**Title: Identify the Suitable Alkene for Compound Formation**
**Question:**
What alkene is best used to produce the following compound?
**Reaction Condition:**
- Reagent: \(\text{H}_2\text{O}/\text{H}_2\text{SO}_4\)
**Target Compound:**
- Structure: A hexane backbone with an OH group attached to the second carbon.
**Options:**
**Alkenes:**
1. **I:** A hexane chain with a double bond between the second and third carbon atoms.
2. **II:** A hexane chain with a double bond between the third and fourth carbon atoms.
3. **III:** A hexane chain with a double bond between the fourth and fifth carbon atoms.
4. **IV:** A hexane chain with a double bond at the terminal end between the first and second carbon atoms.
**Answer Choices:**
- ○ I
- ○ II
- ○ III
- ○ IV
**Explanation:**
Each option features an alkene with varying positions of the double bond. The reaction involves the hydration of an alkene using water and sulfuric acid, leading to alcohol formation according to Markovnikov’s rule, which predicts the addition of water across the double bond so the hydroxyl group attaches to the more substituted carbon.
**Link:** [Additional Resources](#)
**Note:** Understanding the mechanism of electrophilic addition will help determine the correct alkene choice for producing the target alcohol.
Expert Solution
Step 1
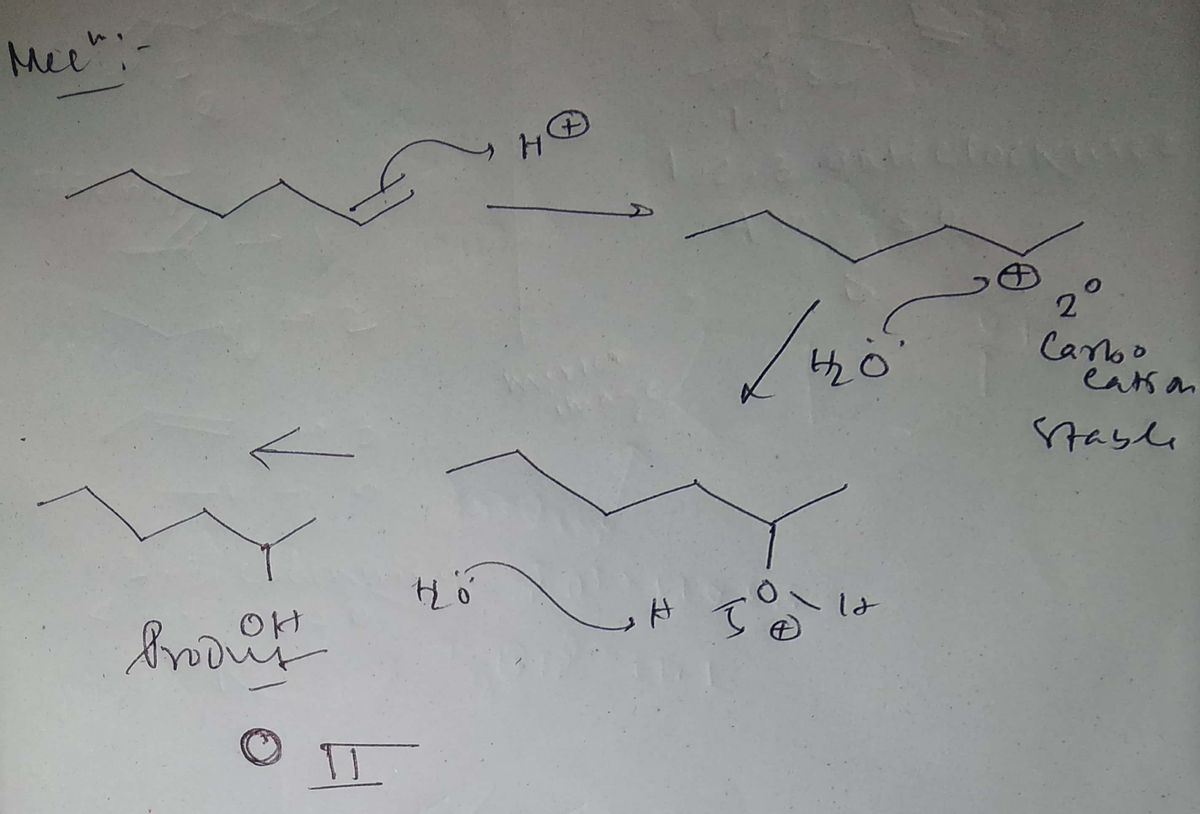
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY