Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![Chlorine dioxide, ClO₂, is prepared by treating sodium chlorite with chlorine gas.
The chemical equation for the reaction is:
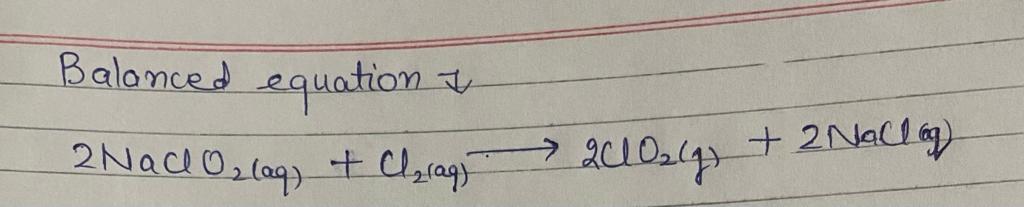
\[ 2 \text{NaClO}_2(\text{aq}) + \text{Cl}_2(\text{g}) \rightarrow 2 \text{ClO}_2(\text{g}) + 2 \text{NaCl}(\text{aq}) \]
**Question:** How many grams of chlorine dioxide can be produced from 2.75 moles of sodium chlorite?
**Answer:** [Input field for the answer]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fa6863c8d-f09e-486e-9969-ee798cd8c2b9%2F177f9143-48d9-48dd-b38d-b5efed6e422c%2Fr9wedfr_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Chlorine dioxide, ClO₂, is prepared by treating sodium chlorite with chlorine gas.
The chemical equation for the reaction is:
\[ 2 \text{NaClO}_2(\text{aq}) + \text{Cl}_2(\text{g}) \rightarrow 2 \text{ClO}_2(\text{g}) + 2 \text{NaCl}(\text{aq}) \]
**Question:** How many grams of chlorine dioxide can be produced from 2.75 moles of sodium chlorite?
**Answer:** [Input field for the answer]
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY