Typical activity (mCca, where Procedure, isotope 1 mCi= 3.7x10' Bq Brain scan 7.5 11 In 7.5 1"C PET) 20 13N (PET) 20 150 PET) 50 18F (PET) 10 Lung scan 133 Xe 7.5 Cardiovascular blood pool 1311 0.2 Cardiovascular arterial flow 201 TI 24 Na 7.5 Thyroid scan 1311 0.05 1231 0.07 Liver scan 198 Au (colloid) 0.1 Te (colloid) Bone scan 0.1 10 Kidney scan 197 Hg 0.1 1.5
Radioactive decay
The emission of energy to produce ionizing radiation is known as radioactive decay. Alpha, beta particles, and gamma rays are examples of ionizing radiation that could be released. Radioactive decay happens in radionuclides, which are imbalanced atoms. This periodic table's elements come in a variety of shapes and sizes. Several of these kinds are stable like nitrogen-14, hydrogen-2, and potassium-40, whereas others are not like uranium-238. In nature, one of the most stable phases of an element is usually the most prevalent. Every element, meanwhile, has an unstable state. Unstable variants are radioactive and release ionizing radiation. Certain elements, including uranium, have no stable forms and are constantly radioactive. Radionuclides are elements that release ionizing radiation.
Artificial Radioactivity
The radioactivity can be simply referred to as particle emission from nuclei due to the nuclear instability. There are different types of radiation such as alpha, beta and gamma radiation. Along with these there are different types of decay as well.
The activities of 131 I and 123 I used in thyroid scans are
given in Table 32.1 to be 50 and 70 μCi , respectively. Find
and compare the masses of 131 I and 123 I in such scans,
given their respective half-lives are 8.04 d and 13.2 h. The
masses are so small that the radioiodine is usually mixed with
stable iodine as a carrier to ensure normal chemistry and
distribution in the body.

The expression to determine the mass is,
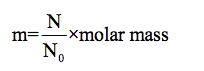
The mass of 131I
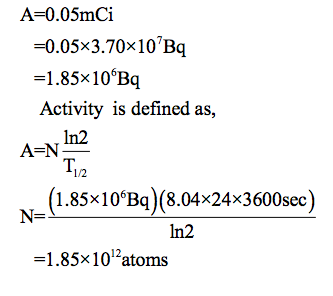
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images









