Theoretical and Excess Air One hundred mollh of butane (Cy this and of fed to a Combustion reactor. Calculate the percent excers air.
Theoretical and Excess Air One hundred mollh of butane (Cy this and of fed to a Combustion reactor. Calculate the percent excers air.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
chemical processes

Transcribed Image Text:**Title: Theoretical and Excess Air Calculation in Combustion Processes**
**Description:**
This page explains how to calculate theoretical and excess air in combustion reactions, specifically using the example of butane combustion.
**Example Problem:**
- *Given:*
- 100 mol/h of butane (C₄H₁₀)
- 500 mol/h of air are fed to a combustion reactor.
**Task:**
- Calculate the percent excess air.
**Additional Note:**
- Be clear to show how 476 mol air was obtained in calculations.
This example demonstrates the steps involved in determining the percentage of air that exceeds the theoretical requirement for complete combustion, enabling more effective and efficient reactor design and operation.
Expert Solution
Step 1: Given data
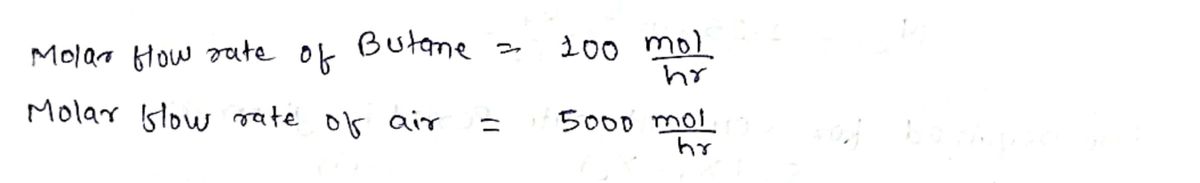
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The