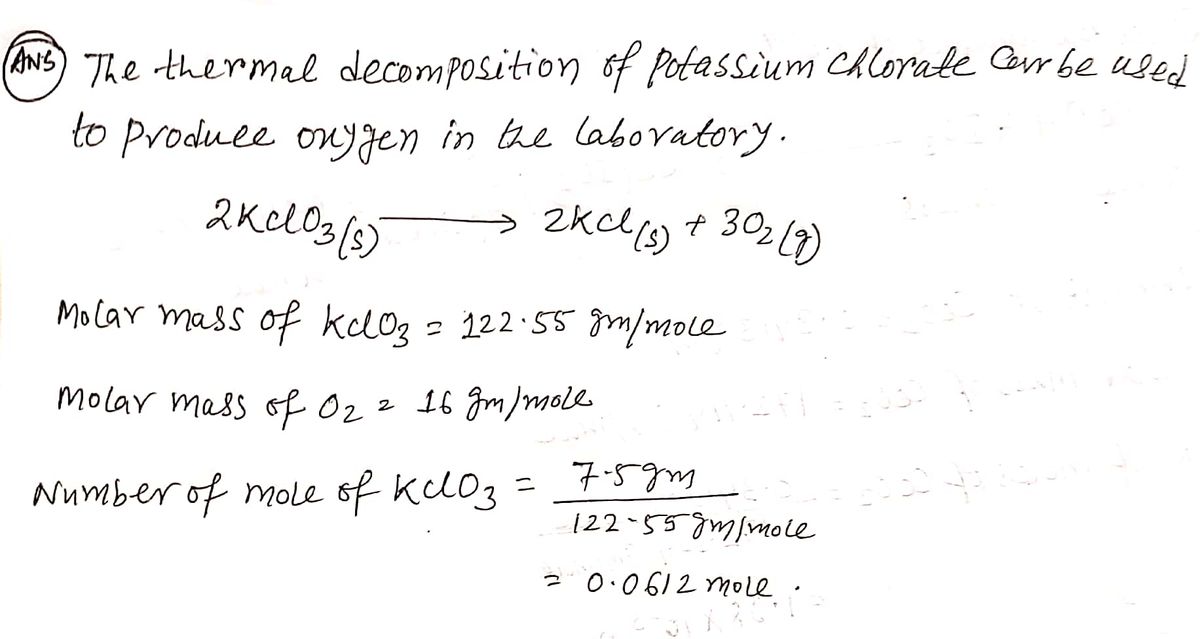
The thermal decomposition of potassium chlorate can be used to produce oxygen in the laboratory. 2KCIO3(s)>> 2KCI (s) + 3O2(g) What volume (L) of O2 gas at 25oC and 1.00 atm pressure is produced by the decomposition of 7.5 g of KCIO3(s)?
The thermal decomposition of potassium chlorate can be used to produce oxygen in the laboratory. 2KCIO3(s)>> 2KCI (s) + 3O2(g) What volume (L) of O2 gas at 25oC and 1.00 atm pressure is produced by the decomposition of 7.5 g of KCIO3(s)?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter20: Chemistry Of Hydrogen, Elements In Group 3a Through 6a, And The Noble Gases
Section: Chapter Questions
Problem 20.65QE
Related questions
Question
The thermal decomposition of potassium chlorate can be used to produce oxygen in the laboratory.
2KCIO3(s)>> 2KCI (s) + 3O2(g)
What volume (L) of O2 gas at 25oC and 1.00 atm pressure is produced by the decomposition of 7.5 g of KCIO3(s)?
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning