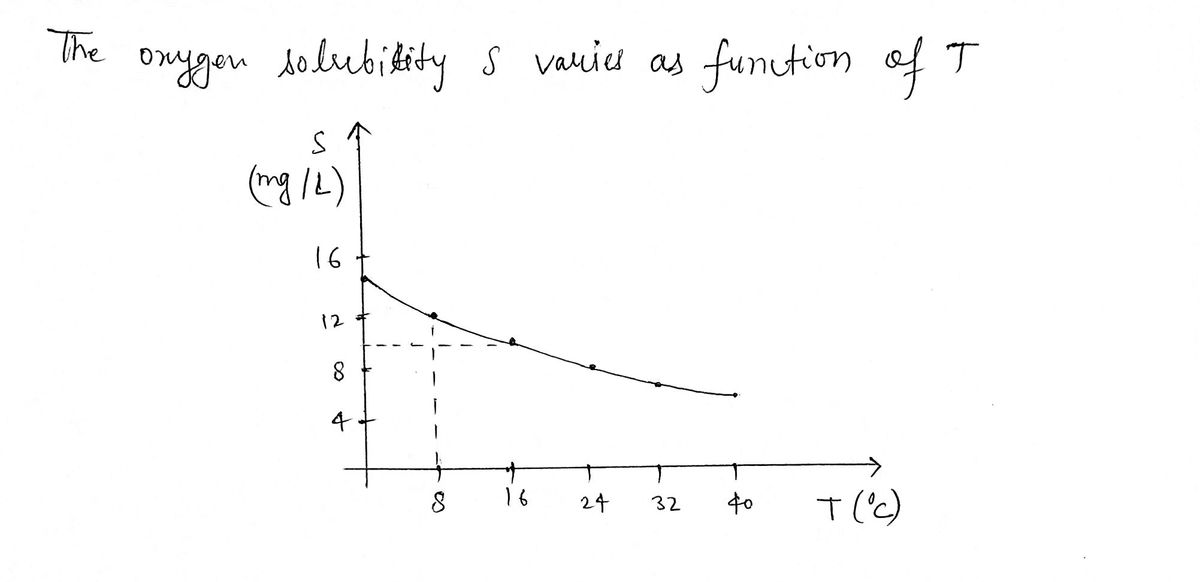
The quantity of oxygen that can dissolve in water depends on the temperature of the water. (So thermal pollution influences the oxygen content of water.) The graph shows how oxygen solubility S varies as a function of the water temperature T. SA (mg/L) 16+ 12 8+ 4 8 16 24 32 40 T(°C) (a) What is the meaning of the derivative S '(T)? OS '(T) is the rate at which oxygen solubility changes with respect to the water temperature. OS '(T) is the rate at which temperature changes with respect to the oxygen solubility. OS (T) is the rate at which temperature changes with respect to the temperature. OS (T) is the rate at which oxygen solubility changes with respect to the oxygen solubility. OS '(T) is the current level of oxygen solubility for a given temperature. (b) Estimate the value of S '(8). (Round your answer to three decimal places.) S'(8) = (mg/L)/°C
The quantity of oxygen that can dissolve in water depends on the temperature of the water. (So thermal pollution influences the oxygen content of water.) The graph shows how oxygen solubility S varies as a function of the water temperature T. SA (mg/L) 16+ 12 8+ 4 8 16 24 32 40 T(°C) (a) What is the meaning of the derivative S '(T)? OS '(T) is the rate at which oxygen solubility changes with respect to the water temperature. OS '(T) is the rate at which temperature changes with respect to the oxygen solubility. OS (T) is the rate at which temperature changes with respect to the temperature. OS (T) is the rate at which oxygen solubility changes with respect to the oxygen solubility. OS '(T) is the current level of oxygen solubility for a given temperature. (b) Estimate the value of S '(8). (Round your answer to three decimal places.) S'(8) = (mg/L)/°C
Calculus: Early Transcendentals
8th Edition
ISBN:9781285741550
Author:James Stewart
Publisher:James Stewart
Chapter1: Functions And Models
Section: Chapter Questions
Problem 1RCC: (a) What is a function? What are its domain and range? (b) What is the graph of a function? (c) How...
Related questions
Question

Transcribed Image Text:The quantity of oxygen that can dissolve in water depends on the temperature of the water. (So thermal pollution influences the oxygen
content of water.) The graph shows how oxygen solubility S varies as a function of the water temperature T.
SA
(mg/L)
16+
12-
8
4
0
8 16 24 32 40 T(°C)
(a) What is the meaning of the derivative S '(T)?
OS (T) is the rate at which oxygen solubility changes with respect to the water temperature.
OS '(T) is the rate at which temperature changes with respect to the oxygen solubility.
OS '(T) is the rate at which temperature changes with respect to the temperature.
OS '(T) is the rate at which oxygen solubility changes with respect to the oxygen solubility.
OS (T) is the current level of oxygen solubility for a given temperature.
(b) Estimate the value of S '(8). (Round your answer to three decimal places.)
S '(8) =
(mg/L)/°C
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Calculus: Early Transcendentals
Calculus
ISBN:
9781285741550
Author:
James Stewart
Publisher:
Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:
9780134438986
Author:
Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:
PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:
9780134763644
Author:
William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:
PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:
9781285741550
Author:
James Stewart
Publisher:
Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:
9780134438986
Author:
Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:
PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:
9780134763644
Author:
William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:
PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:
9781319050740
Author:
Jon Rogawski, Colin Adams, Robert Franzosa
Publisher:
W. H. Freeman


Calculus: Early Transcendental Functions
Calculus
ISBN:
9781337552516
Author:
Ron Larson, Bruce H. Edwards
Publisher:
Cengage Learning