The photo below contains the question thank you.

The weight of the content in the test tube before heating gives the collective weight of KClO3 and MnO2 whereas the weight of the content in the test tube after heating gives the collective weight of KCl and MnO2.
Weight of test tube = 34.38
Weight of the content before heating = 61.88 – 34.38 g = 27.50 g
Weight of the content after heating = 60.7 – 34.38 g = 26.32 g
Volume of oxygen gas released (V) = volume of water displaced = 1.445 L
Pressure of the moist gas = 752.1 mm Hg = 0.989 atm
Vapor pressure of water = 31 torr = 0.041 atm
Pressure of dry gas = Pressure of moist gas – Vapor pressure of water
Pressure of dry gas (P) = 0.989 atm – 0.041 atm = 0.948 atm
Temperature of oxygen gas (T) = 298.3 K
Universal gas constant R = 0.0821 Latm/molK
The number of moles of gas (n) can be calculated by using the ideal gas equation PV = nRT.
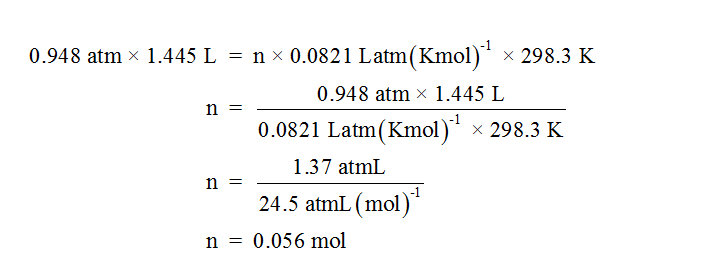
Step by step
Solved in 2 steps with 1 images









