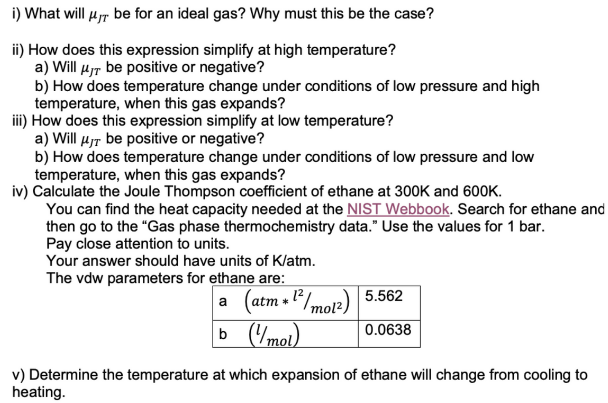
The expression for the Joule Thompson coefficient for a gas described by the van der Waals equation of state, at low pressures, can be expressed as: 1 2a = HJT —— (12²0 T - b) Cp\R*T i) What will be for an ideal gas? Why must this be the case? ii) How does this expression simplify at high temperature? a) Will μ be positive or negative? b) How does temperature change under conditions of low pressure and high temperature, when this gas expands? iii) How does this expression simplify at low temperature? a) Will μ be positive or negative? b) How does temperature change under conditions of low pressure and low temperature, when this gas expands? iv) Calculate the Joule Thompson coefficient of ethane at 300K and 600K. You can find the heat capacity needed at the NIST Webbook. Search for ethane an then go to the "Gas phase thermochemistry data." Use the values for 1 bar. Pay close attention to units. Your answer should have units of K/atm. The vdw parameters for ethane are: a (atm * 1²/mol²) b (1/mol) 5.562 0.0638 v) Determine the temperature at which expansion of ethane will change from cooling to heating.
The expression for the Joule Thompson coefficient for a gas described by the van der Waals equation of state, at low pressures, can be expressed as: 1 2a = HJT —— (12²0 T - b) Cp\R*T i) What will be for an ideal gas? Why must this be the case? ii) How does this expression simplify at high temperature? a) Will μ be positive or negative? b) How does temperature change under conditions of low pressure and high temperature, when this gas expands? iii) How does this expression simplify at low temperature? a) Will μ be positive or negative? b) How does temperature change under conditions of low pressure and low temperature, when this gas expands? iv) Calculate the Joule Thompson coefficient of ethane at 300K and 600K. You can find the heat capacity needed at the NIST Webbook. Search for ethane an then go to the "Gas phase thermochemistry data." Use the values for 1 bar. Pay close attention to units. Your answer should have units of K/atm. The vdw parameters for ethane are: a (atm * 1²/mol²) b (1/mol) 5.562 0.0638 v) Determine the temperature at which expansion of ethane will change from cooling to heating.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question

Transcribed Image Text:The expression for the Joule Thompson coefficient for a gas described by the van der
Waals equation of state, at low pressures, can be expressed as:
1 2a
-—- (2017-b)
Cp\R* T
i) What will μT be for an ideal gas? Why must this be the case?
ii) How does this expression simplify at high temperature?
a) Will μT be positive or negative?
b) How does temperature change under conditions of low pressure and high
temperature, when this gas expands?
iii) How does this expression simplify at low temperature?
a) Will μT be positive or negative?
b) How does temperature change under conditions of low pressure and low
temperature, when this gas expands?
iv) Calculate the Joule Thompson coefficient of ethane at 300K and 600K.
HJT
=
You can find the heat capacity needed at the NIST Webbook. Search for ethane and
then go to the "Gas phase thermochemistry data." Use the values for 1 bar.
Pay close attention to units.
Your answer should have units of K/atm.
The vdw parameters for ethane are:
a
(atm*1²/mo12) 5.562
b (1/mol)
0.0638
v) Determine the temperature at which expansion of ethane will change from cooling to
heating.
Expert Solution
Step 1
Given:
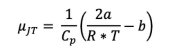
To find:
Step by step
Solved in 7 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY