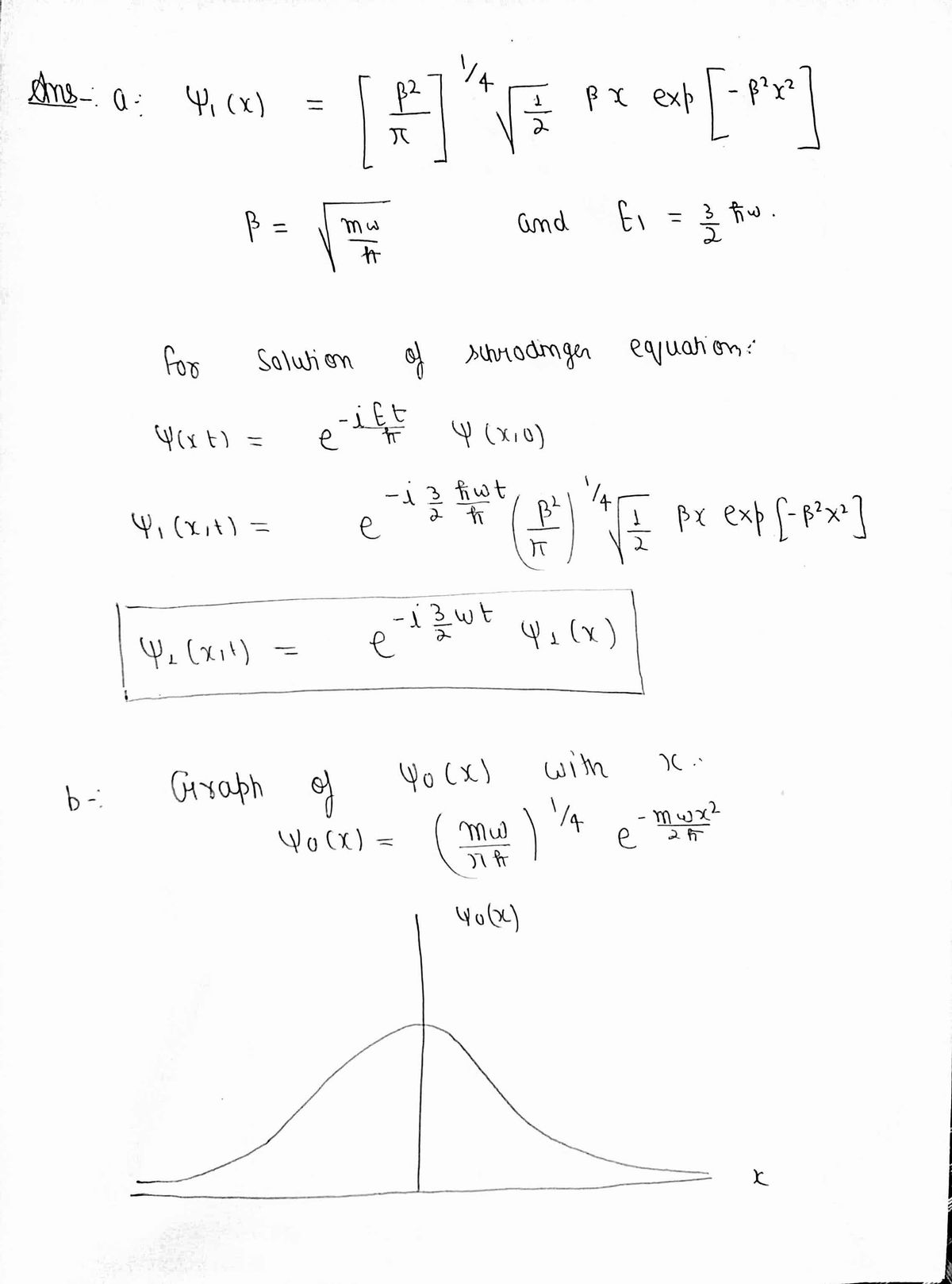
The eigenfunction for OHS for n=1 is of the form W1(x) exp(-p?x?) with value - and energy E = hw Ima %3D a. Write the form of the function as a solution of the Schrodinger equation for this OHS (y(x,t)) b. Draw the wave function and energy levels of this OHS until n = 4.
Q: 2. A car of mass 1200 kg initially moving at a speed of 10m/s suddenly changes its speed to 15m/s.…
A: a) The required work done is,
Q: Soap bubbles can be produces when pressure outside and inside of the bubble is different. Soap…
A: Given: ρa=1.29kg/m3, ρh=0.09kg/m3, m=5mg Let the volume of the bubble be V. The air exerts a buoyant…
Q: -component of the net force component of the net force
A: Given: Mass of standard body is 1 kg F1=(2.6 N)i+(3.8 N)j F2=(-2.4 N)i+(-6 N)j
Q: 2 0/1 point Current flows through two parallel wires placed 15 cm apart. The magnitude of the…
A:
Q: 6.10* A thick glass lens of index 1.50 has radii of +23 cm and +20 cm, so that both vertices are to…
A:
Q: 2. Determine the velocity of the 60-lb block A if the two blocks are released from rest and the…
A:
Q: A solenoid of length 1.00 cm and radius 0.850 cm has 25 turns. If the wire of the solenoid has 1.45…
A: Given: In this question, the given details are A solenoid of length 1.00 cm and the radius 0.850 cm…
Q: Four objects (A, B, C, & D) make up a closed system. The table below shows their energy at various…
A: Given data: Four points make a closed system at t = 0 s the energy at various points EA = 59 J,…
Q: Miley came in with a wrecking ball A with a mass of 500 kg. It is released from rest from height h…
A: Since you have posted a question with multiple subparts, we will solve first three as per Q&A…
Q: (!) THE FOLLOWING QUESTIONS ARE BASED ON THE INFORMATION GIVEN BELOW. vo An electron at point A as…
A: Given: v0=1.7×106m/sl=5cmme=9.109×10-31kgqe=1.6×10-19C
Q: Can someone look at the options again. Please select all of the correct answers.
A:
Q: 1. a) What is the Potential Energy of a 30kg mass at the edge of a roof 110 m above the ground? The…
A:
Q: What formulas did you use?
A:
Q: Click on the arrow which best represents the force on the lower (pink) charged sphere
A: Arrow pointing towards right is the best fit.
Q: What is the difference between Charles law of gas, and Gay-Lussac's law of gas?. If possible can it…
A: This problem is from thermodynamics. Please have a good look
Q: In the figure, a long straight wire carries a current i = 28.6 A and a rectangular loop carries…
A: The force between the two current carrying conductor carrying current is given by F = μo4π2 i i'rL…
Q: Why is Mathematics an important tool in the study of physics?
A: A question often asked i.e., the importance of mathematics in physics. Some people think that…
Q: u please provide clear answer, you can write it down on
A: The term acceleration refers to any process in which velocity changes. In physics, velocity refers…
Q: 1.) A 30N force acts at a 40° angle from the horizontal on a 30kg block at rest on a perfectly…
A: Given, F=30 Nθ=400m=30 kgd=7.2 m
Q: 8 B B 8 B 1. One positively charged object, and one neutral object. 2. One negatively charged…
A: Electric field and lines due to charges.
Q: Two equally charged particles repel each other with a force F. If their separation is doubled, they…
A: Coulomb's law : The electric force between charged bodies at rest is conventionally called…
Q: Calculate the average force a bumper would have to exert to bring a 1200-kg car to rest in 15 cm…
A: Given: In this question, the given values are, The given mass value is, m=1200kg. The value of the s…
Q: Figure below shows an object's velocity vs time graph. Draw the position vs time graph. Assume x = 0…
A: For the segment "A" velocity is constant. Since velocity is constant(acceleration =0), displacement…
Q: Fill in the missing number. 123 1 2 ?
A: We have to find the missing number in the following series. 1 2 3 1 2 ?
Q: What is the opposite of squaring a number? O divide O multiply O fractions square root
A:
Q: Geothermal power plants use the energy of underground fluids for the production of electricity. The…
A: Required : Environmental effects of geothermal plants.
Q: the ball does
A: Given: Vertical angle of string is 37o
Q: Direction: Identify the following properties through their given units and convert them to SI units.…
A:
Q: Compute the coordinates of the centroid (T, j) of the area shown. d C a Values for dimensions on the…
A:
Q: O Gc and K1c have different units and are related to each other by the tensile modulus. The relative…
A: The question contains 6 statements related to the "Introduction to Fracture Mechanics" where we need…
Q: 14) A steel tape has markings every 1/10 of a centimeter. Yet your classmate reported a measurement…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Wrong answer for part (a), please provide correct answer, please. thank you!
A:
Q: Question 1: What is the relationship between the Torque and the distance of the force from the axis…
A: 1. Torque is defined as a force applied to a point on an object about the axis of rotation. The…
Q: In the case of half-wave rectifier, what is the correct answer:
A: As per the company policy, only 1st question will be answered here, please post the rest separately.…
Q: Capacitance of a Thundercloud The charge center of a thundercloud, drifting 3.0 km above the earth's…
A: Given: In this question, the given details are, Capacitance of a Thundercloud The charge center of a…
Q: 8.) A 500 g copper rod is at a temperature of 7 °C. It is heated using 2.5 x 10 J of energy. What is…
A: As per our guidelines, we are supposed to answer only first one question in case of multiple…
Q: Construct circuits from inverters, AND gates, and OR gates for each of the following boolean…
A:
Q: QUESTIONS AND PROBLEMS Two identical charges, Q, and Q2 exert a force Fg on one another. What will…
A:
Q: A loop is oriented as shown below inside a region of uniform and constant external magnetic field.…
A: Given that---- initial angle = 0° final angle = 180 ° Question ❓ then rotated by 180° around the…
Q: Question 2 NOTE: Round off answer up to 2 decimal places. Use g = 9.8 m/s². Indicate the unit…
A:
Q: A block is tied to a spring and is oscillating with an amplitude of A = 16 cm. The angular frequency…
A: Given: The given values are, The given amplitude value is, A=16 cmA=16×10-2m And then the angular…
Q: KI Ny
A:
Q: A ball is initially at a height of 10m above the ground. It rolls down a slope with an initial speed…
A: From conservation of energy, the expression for the final speed is,
Q: Show how to make the following gates using only NOR gates: a) an INVERT from NOR
A: a) INVERT gate is made by joining inputs of NOR gate. b) AND gate is made by using 3 NOR gates. Two…
Q: A solid cylindrical conducting shell of inner radius a = 5.8 cm and outer radius b = 7.4 cm has its…
A: To determine: (1) The y-component of magnetic field at point P. Let there be a loop at 24 cm or…

Step by step
Solved in 3 steps with 3 images
