The dew pressure for the system Water-Acetic Acid at y 0.6 is (kPa)? 210 3.02 9.5 3.2 O 21
The dew pressure for the system Water-Acetic Acid at y 0.6 is (kPa)? 210 3.02 9.5 3.2 O 21
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:The following is Pxy diagrams for the four systems at T = 25°C:
A) Benzene-Formaldehyde, B) Benzene-Diethyl ketone, C) Water-Acetic acid, and D) Water Formic acid
600.00
Benzene - Formaldehyde
s00.00-
12.00-
Benzene- Diethyl Ketone
400.00
10.00
200.00
8.00
200.00-
100.00-
4.00
4.00
0.00
020
100
0.00
020
0.40
00
1.00
x Benzene
x Benzene
3.40
Water + Acetic acid
00
Water-Formid acid
3.20
3.30
200
5.00
230-
450
260
400
240
3.50
220
3.00
200
250
00
020
CAO
G.0
1,00
00
0.20
0.40
0.00
0.00
1.00
x H20
Pressure Pa)
Pressure OPa)

Transcribed Image Text:x Benzene
3.40
Water Acetic acid
1.20-
600
Water-Formic acid
5.50-
3.00-
5.00
2.00
4.50
280
4.00-
2.50 -
2.20
300 -
200
00
020
2.50
100
x H20
00
020
O.40
000
100
XH20
Q Zoom image
The dew pressure for the system Water-Acetic Acid at y = 0.6 is (kPa)?
O 210
3.02
O 9.5
O 3.2
O 21
Pressure (Pa)
Expert Solution
Step 1
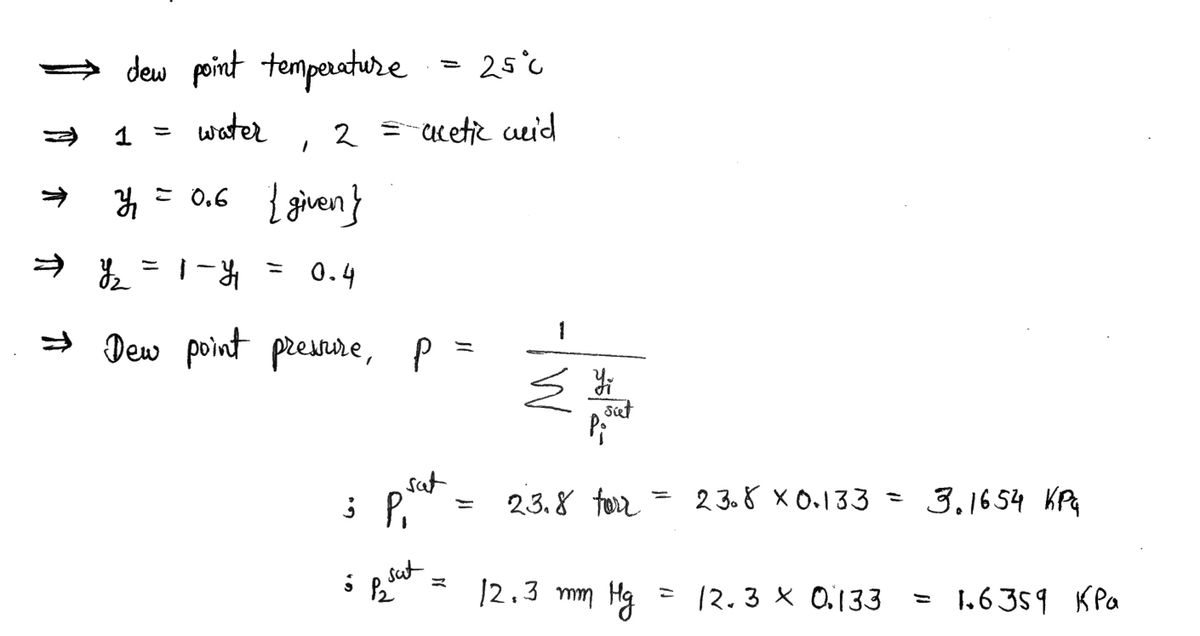
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The