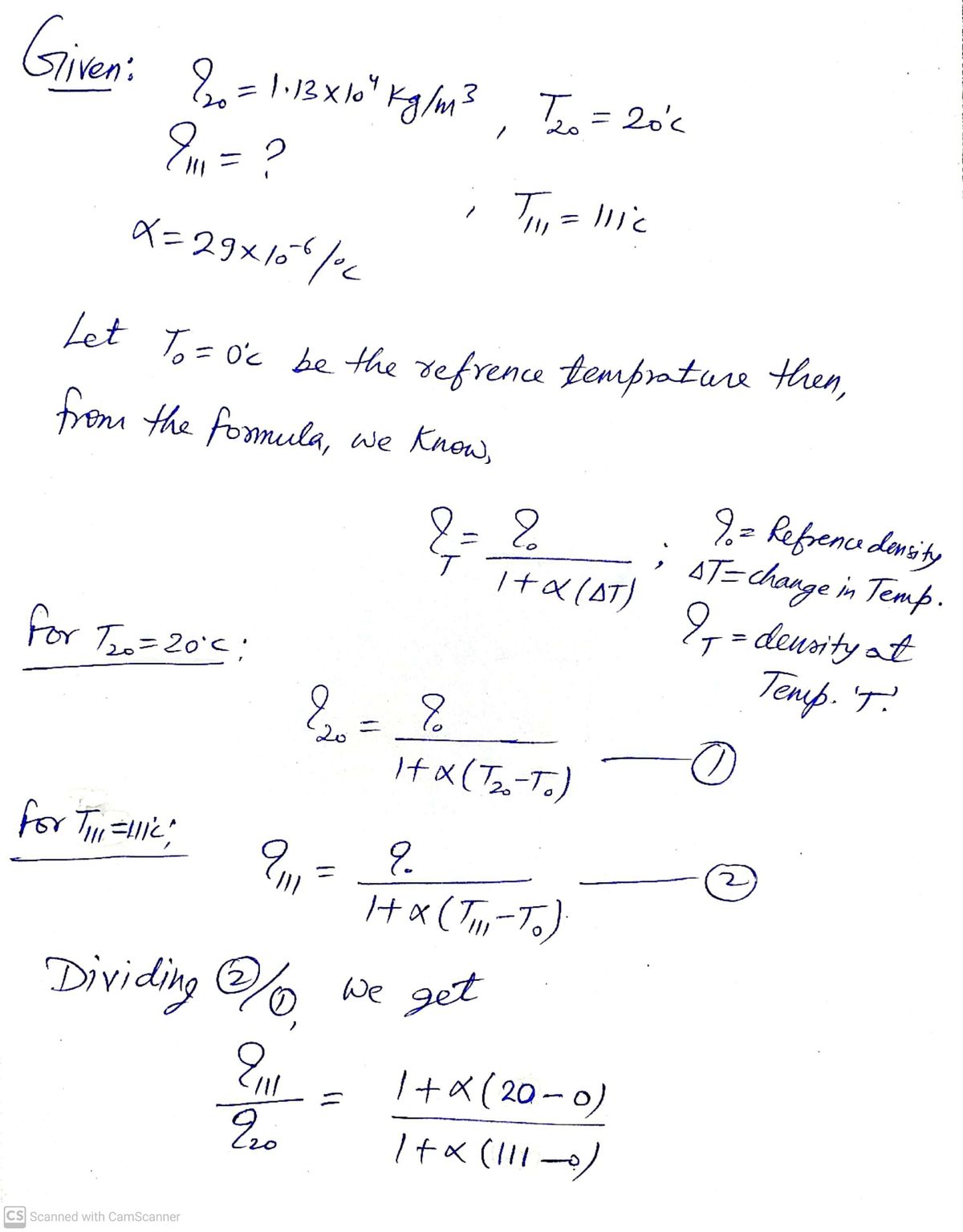
The density of lead is 1.13 x 10* kg/m at 20.0°C. Find its density (in kg/m3) at 111°C. (Use a = 29 × 10-6 (oC)-1 for the coefficient of linear expansion. Give your answer to at least four significant figures.) HINT kg/m3 Need Help? Read It Watch It
Q: a 100 mL glass beaker is filled to the brim with ethyl alcohol at a temperature of 7.50°C, how much…
A:
Q: Find the root mean square speed of oxygen molecules in this room. (An oxygen molecule is diatomic.…
A:
Q: The density of lead is 1.13 x 104 kg/m³ at 20.0°C. Find its density (in kg/m³) at 115°C. (Use a = 29…
A:
Q: If a 100 mL glass beaker is filled to the brim with ethyl alcohol at a temperature of 5.20°C, how…
A:
Q: If 3 47 m³ of a gas initially at STP is placed under a pressure of 3 47 atm the temperature of the…
A:
Q: The density of lead is 1.13 x 104 kg/m3 at 20.0°C. Find its density (in kg/m3) at 135°C. (Use a = 29…
A:
Q: A solid aluminum sphere has a radius of 14.3 cm at a certain temperature. It is then cooled by 64.4…
A: According to question we need to find new Volume?
Q: What is the change in length of a 3.00-cm-long column of mercury if its temperature changes from…
A: Given data: Length of mercury column(L)= 3.00 cm = 30.0 mm Initial temperature (Ti) = 39.0° Final…
Q: A large cylindrical tank contains 0.850 m' of nitrogen gas at 26.0°C and 6.50×103 Pa (absolute…
A:
Q: If you have a rigid 212 mL container holding 1.16 X 10^18 atoms of helium. What is the pressure In…
A:
Q: An empty cylindrical canister 1.60 m long and 87.0 cm in diameter is to be filled with pure oxygen…
A: Solution:-Given thatlength of canister (l)=1.6 mdiameter (d)=87 cm=0.87 mTemperature…
Q: A wire is 29.5 m long at 3.50°C and is 1.60 cm longer at 35.5°C. Find the wire's coefficient of…
A: Answer : 1.69 ×10-5 ⁰C-1
Q: A 10.00L tank at −5.9°C is filled with 8.34g of boron trifluoride gas and 18.0g of sulfur…
A:
Q: A metal rod 0.70m is heated from 20℃ to 80℃. The length of the rod increases by 0.82mm. What is the…
A:
Q: A 1000.0 mL glass beaker is filled to the top with water. The filled beaker is placed onto a heating…
A:
Q: If 3.8 molesof an ideal gas is at a temperature of65°Cand contained in a0.27ˍm³volume, then what…
A:
Q: The gauge pressure in your car tires is 3.94 x 10° N/m² at a temperature of 39.0°C when you drive it…
A: By ideal gas law PV = nRT Here, V,n and R are constant T2=73-38.8 KT1=273+39 K
Q: 7. A container holds 5.00kg of oxygen (0,) gas in a volume of V =8.25x10m²at room temperature, T…
A: The molar mass of oxygen, O2
Q: 3- A bubble of air rises from the bottom of a lake, where the pressure is 3.03x102 Pa, to the…
A:
Q: The density of gasoline is 7.30 x 10² kg/m³ at 0°C. Its average coefficient of volume expansion is…
A:
Q: Each molecule of the diatomic gas carbon monoxide contains one carbon atom and one oxygen atom. If…
A:
Q: 0.10 mol of Argon gas is admitted to an evacuated 50cc container at 20°C. The gas is then heated at…
A: Given data The initial volume is V1=50 cm3 The initial temperature is T1=20oC The final temperature…
Q: A tank of compressed air of volume 1.00 m³ is pressurized to 30.5 atm at T = 273 K. A valve is…
A:
Q: 1.5 The table below lists the observed values of the pressure P of a gas in a constant-volume gas…
A:
Q: A steel bar and brass bar have the exact same length of 1.8000 m at -15.00 deg C. The coefficient of…
A: we first need to find the change in length of each bar as the temperature increases from -15.00°C to…
Q: The density of lead is 1.13 x 104 kg/m at 20.0°C. Find its density (in kg/m) at 118°C. (Use a = 29 x…
A: Ans is 1.1204×10^4 kg/m^3
Q: The density of lead is 1.13 ✕ 104 kg/m3 at 20.0°C. Find its density (in kg/m3) at 134°C. (Use ? = 29…
A: The change in volume of a metal occurring due to a change in temperature is given by the equation…
Q: 0.52 mol of argon gas is admitted to an evacuated 3.00 liter (3.00 × 10-3 m3) container at 20.0°C.…
A: Page 1
Q: An arctic weather balloon is filled with 49.6L of helium gas inside a prep shed. The temperature…
A: Initial volume = 49.6 L Initial temperature = 11° C = 284 Kelvin Final temperature = - 39°C = 234…
Q: A lead rod has a length of 60.6 m when the temperature is 12.4 degrees celsius. What is its length…
A:
Q: A3] a) Find the temperature of 1 mole of oxygen molecules if they occupy a volume of 4 L and have a…
A: Hi! Thank you for the question. As per honor code, we are allowed to answer three sub-parts at a…

Step by step
Solved in 2 steps with 2 images

- a rod of alloy metal measures 3.521 m long at 290 degrees celius . At 373 degrees celcius the rod is 3.523 m long . Determine the value of the coefficient of linear expansion for the metal .The area A of a rectangular plate is ab = 1.4 m?. Its coefficient of linear expansion is a = 22 x 10° /C°. After a temperature rise AT = 17°C, side a is longer by Aa and side b is longer by Ab (see the figure). Neglecting the small quantity (AaAb)/ab, find AA. %3D a Да Aa · Ab AbSuppose you have 0.53 moles of an ideal gas. What is the average kinetic energy of that gas (in units of kJ) if the temperature of the gas is 280.2K? Note: It is understood that the unit of your answer is some number of kilo-Joules, however do not explicitly include units in your answer. Enter only a number. If you do enter a unit ("kJ" in this case), you answer will be counted wrong.
- A wire lengthens 4% due to thermal expansion when heated from T. to T. If the wire were bent into the shape of a square, what percent increase in length would you expect along one edge of the square? If the wire were bent into the shape of a circle, what percent increase in diameter would you expect? Explain, using sentences and diagrams.I Review I Cons A weather balloon rises through the atmosphere, its volume expanding from 3.6 m to 12 m3 as the temperature drops from 20 °C to -10 °C. Part A For the steps and strategies involved in solving a similar problem, you may view a Video Tutor Solution. If the initial gas pressure inside the balloon is 1.0 atm, what is the final pressure? Express your answer in atmospheres. n ΑΣφ ? P = atm Submit Request AnswerHelp please
- Amanda discovers that the gauge pressure in her car tires is 2.50 ×105 Pa at a temperature of 35.0oC. The average volume of a single tire at that temperature is 10.0 L. 1.) Find how many moles of gas (air) are in one of Steve’s tires. 2.) Later Amanda travels to a place where the temperature is –40.0oF. She notices that the tires are looking low and estimates that each tire has lost approximately 1.0 L of its volume. What is the gauge pressure in the tire now?An ideal gas at 7°C is in a spherical flexible container having a radius of 1.04 cm. The gas is heated at constant pressure to 88°C. Determine the radius of the spherical container after the gas is heated. [Volume of a sphere = (4/3)?r3.]The density of lead is 1.13 x 10“ kg/m³ at 20.0°C. Find its density (in kg/m) at 131°C. (Use a = 29 x 10-° (°C)-- for the coefficient of linear expansion. Give your answer to at least four significant figures.) HINT 3 × kg/m³ 11216.1