The bond energies of Cl-Cl, C-C and C-H are 59 Kcal/mole, 90 Kcal/mole and 105 Kcal/mole respectively. Considering the reaction of ethane with Cl2, which of the following statements are correct?
The bond energies of Cl-Cl, C-C and C-H are 59 Kcal/mole, 90 Kcal/mole and 105 Kcal/mole respectively. Considering the reaction of ethane with Cl2, which of the following statements are correct?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.61PAE: 4.61 What is actually measured by the octane ratings of different grades of gasoline?
Related questions
Question
The bond energies of Cl-Cl, C-C and C-H are 59 Kcal/mole, 90 Kcal/mole and 105 Kcal/mole respectively. Considering the reaction of ethane with Cl2, which of the following statements are correct?
options:
|
|
A. When heat is the source of energy, Cl-Cl bond is the easiest to break |
|
|
B. When light is the source of energy, Cl-Cl absorbs visible light, but ethane does not |
|
|
C. If heat is the source of energy, the C-H bonds breaks easily compared to other bonds involved in the reaction |
|
|
D. If heat is the source of energy, C-C bonds breaks easily compared to other bonds involved in the reaction |
|
|
Both A & B |
Expert Solution
Step 1
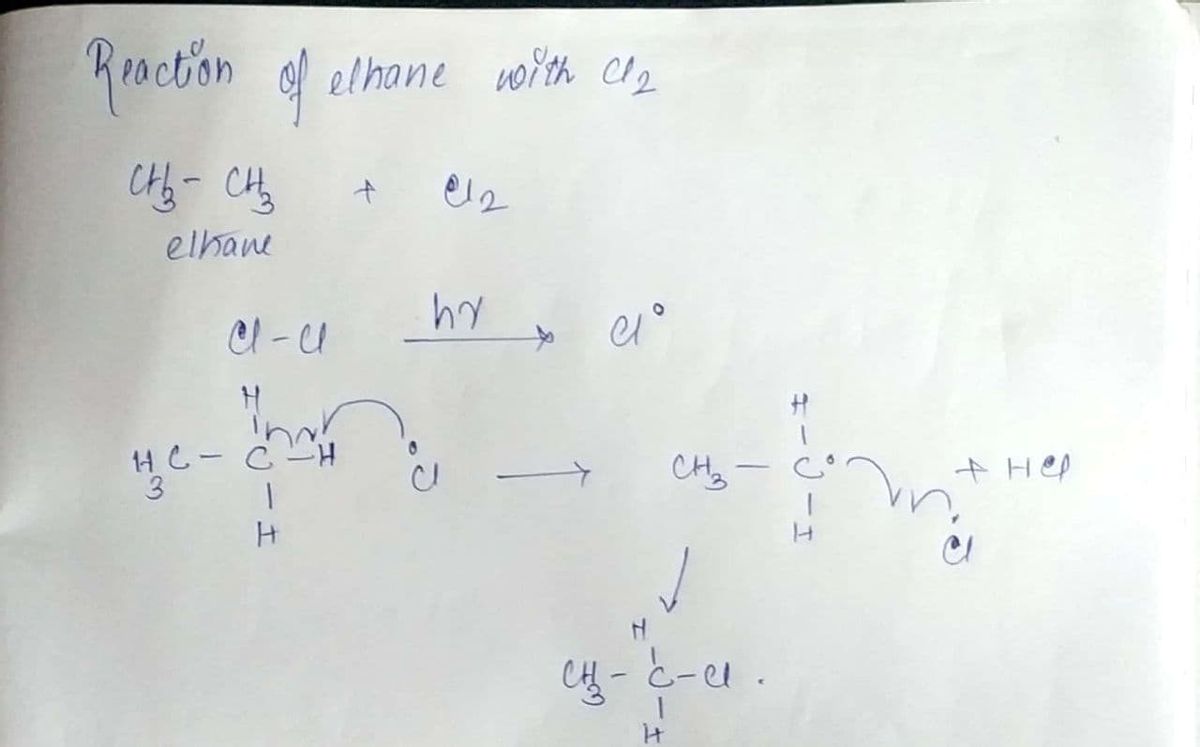
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning