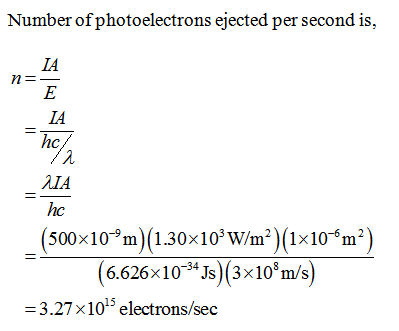
The binding energy of sodium is 2.28 eV. Calculate the number of photoelectrons ejected from a 1.00 mm^2 area of Sodium metal by 500-nm electromagnetic radiation having an intensity of 1.30 kW/m^2. What power is carried away by the electrons?
Q: The work function of a metal is 2.29 x 10-19 J. In a photoelectric effect experiment, calculate the…
A:
Q: Which type of light has the most energy per photon? ultraviolet infrared green visible X-ray
A:
Q: Question in image. Disregard the pen markings plz.
A: Write the expression for stopping potential
Q: The work function for indium is 4.12 eV. (d) If light of energy 7.14 eV is incident on indium, what…
A: Photoelectric effect is the phenomenon of emission of electrons from metals under the influence of…
Q: Most microwave ovens emit electro-magnetic radiation with a wavelength () of 12.24 cm. This EM-…
A: Given: case odf microoven heating water Wavelength = 12.24 cm Rate of photon emission r =…
Q: 3.2 Light with a wavelength of 2.00×10-7 m is directed at a metallic surface made of yttrium, which…
A:
Q: a) Calculate the number of photoelectrons per second ejected from a 0.75-mm² area of sodium metal by…
A: a) The required number of photo-electrons is, n=PE =IAhcλ =IAλhc…
Q: In a photoelectric experiment to determine the work function for a particular metal surface, two…
A: a. From the photo electric effect, write the expression of work function. Substitute the…
Q: What is the stopping potential of an electron that has 7.30 × 10-19 J of kinetic energy in a…
A: Write the expression for the work done. W=qV Here, q is the charge of an electron and V is the…
Q: When ultraviolet light with a wavelength of 354 nm falls on a certain metal surface, the maximum…
A: Given: Ultraviolet light of wavelength λ=354 nm Kinetic energy of photoelectrons…
Q: A strange metallic rock is found and is being tested. Suppose that light with a frequency of 9.60 ✕…
A: Given that Incident frequency f = 9.60 X 1014 Hz Stopping potential V0 = 1.10 V
Q: When ultraviolet light with a wavelength of 374 nm falls on a certain metal surface, the maximum…
A:
Q: Radiation with a frequency of 7.52 × 1014 Hz illuminates a photoelectric surface in a photoelectric…
A: Frequency = 7.52 ×10¹⁴Hz Work function = 2.20 eV
Q: A metal surface is illuminated with light of different wavelengths and the corresponding stopping…
A: Given that a metal surface is illuminated with light of different wavelengths and the corresponding…
Q: The binding energy of Sodium is 2.28eV. Calculate the number of photoelectrons ejected from a…
A:
Q: Q-25: Green light shining on sodium surface causes photoelectrons with maximum kinetic energies of…
A: Given that --- maximum kinetic energies = 0.02 e V. work function of sodium= 2.28 e V, Question ?…
Q: with an energy of 5.3 × 10-19 J strikes an electron in a metal. The binding energy of this metal is…
A:
Q: s) The photoelectric effect can be used to generate power using a solar cell. We will use Aluminum…
A: Given: The work function of the aluminum cathode is ϕ = 4.26 eV The temperature of the sun is T =…
Q: The threshold of dark-adapted (scotopic) vision is 4.5 ✕ 10−11 W/m2 at a central wavelength of 500…
A:
Q: Multiple-Concept Example 3 reviews the concepts necessary to solve this problem. Light is incident…
A: Work function (Φ) = 2.3 eV => Φ = 2.3 × 1.6 × 10-19 J => Φ = 3.68 × 10-19 J Speed of the…
Q: Nhen ultraviolet light with a wavelength of 400 nm falls on a certain metal surface, the maximum…
A:
Q: For a certain metal, the threshold wavelength for the photoelectric effect is 719.0 nm. What is the…
A:
Q: light source is used in a photoelectric effect experiment to determine the work function of a…
A:
Q: An electron in a piece of aluminum requires at least 6.73e-19 J of energy to be ejected from the…
A: "Since all questions are different, as per guidelines we can author only 1st one. Please repost…
Q: The energy conservation principle that applies to the photoelectric experiment is Ephoton =…
A:
Q: You have a sample of Sodium with a Work Function of 2.28 eV that you are shining light upon to…
A:
Q: Ultraviolet radiation of wavelength 121 nm is used to irradiate a sample of potassium metal. The…
A:
The binding energy of sodium is 2.28 eV. Calculate the number of photoelectrons ejected from a 1.00 mm^2 area of Sodium metal by 500-nm
Step by step
Solved in 2 steps with 2 images

- When ultraviolet light with a wavelength of 351 nm falls on a certain metal surface, the maximum kinetic energy of the emitted photoelectrons is measured to be 1.76x10-19 J. How much energy, in Joule, does an electron have to gain in order to escape from the metal surface? Exponential format with 3 SF.The mathematical equation for studying the photoelectric effect is hν = W + 1/2 meu2 where ν is the frequency of light shining on the metal; W is the energy needed to remove an electron from the metal; and me and u are the mass and speed of the ejected electron, respectively. In an experiment, a student found that a maximum wavelength of 351 nm is needed to just dislodge electrons from a metal surface. Calculate the velocity (in m/s) of an ejected electron when the student employed light with a wavelength of 283 nm.Lithium has a work function of 2.51 eV. What is the maximum kinetic energy of the photoelectrons if 9.7 x 10¹4 Hz radiation is incident upon it?
- You set up a photoelectric experiment with an unknown metal to eject electrons. You use light of wavelength λ = 670 nm, which just BARELY ejects electrons from the metal. Planck□s constant is either h = 6.63 x 10-34 J-s or h = 4.14 x 10-15 ev.s. a) What is the binding energy of the unknown metal in eV? ev b) You change the light source to one with a wavelength of λ = 310 nm. Using the binding energy you found in the previous step, find the maximum kinetic energy of an electron that is ejected from the metal in Joules. J c) What is the stopping voltage for an electron with the kinetic energy you just found? VA nickel crystal’s work function is measured to be 5.22 eV at 25°C. As the temperature increases by 300°C, the work function drops by 50 meV. By how much does this shift the threshold wavelength for photoelectric emission?One of the first signs of sunburn is the reddening of the skin (called erythema). As a very rough rule of thumb, erythema occurs if 14.0 mJ of ultraviolet light of approximately 308 nm wavelength (referred to as UVB radiation) is incident on the skin per square centimeter during a single exposure. How many photons are incident on 1.00 cm2 of skin in this amount of exposure? photons
- QUESTION 24 When ultraviolet light with a wavelength of 317 nm falls on a certain metal surface, the maximum kinetic energy of the emitted photoelectrons is measured to be 1.76 x 10-1 J. How much energy, in Joule, does an electron have to gain in order to escape from the metal surface? Exponential format with 3 SF.The work function of a material refers to the minimum energy required to remove an electron from the material. The work function of tungsten is 4.55 eV. Calculate the maximum wavelength (in nm) for the photoelectric emission of electrons. O λ = 220 nm O λ = 287 nm Oλ = 292 nm Oλ = 273 nm