Suppose that attractions are the dominant interaction between gas molecules, and the equation of state is p = nRT/V – n2a/V2. Determine the work (W(non-ideal gas)) of reversible, isothermal expansion of such a gas from initial volume V (initial) = 20.0 L to final volume V(final) = 40.0 L if n = 2.00 mol, T = 300 K, and a = 3.621 atm-L2/mol2. Watch your units. Determine the work (W(ideal gas) of reversible, isothermal expansion of an ideal gas from initial volume V (initial) = 20.0 L to final volume V(final) = 40.0 L if n = 2.00 mol and T = 300 K. Show the difference W(non-ideal) – W(ideal). If all your calculations are done correctly, this result shows you the effect of attractive interaction between gas particles on the work done by the system.
Suppose that attractions are the dominant interaction between gas molecules, and the equation of state is p = nRT/V – n2a/V2.
Determine the work (W(non-ideal gas)) of reversible, isothermal expansion of such a gas from initial volume V (initial) = 20.0 L to final volume V(final) = 40.0 L if n = 2.00 mol, T = 300 K, and a = 3.621 atm-L2/mol2. Watch your units.
Determine the work (W(ideal gas) of reversible, isothermal expansion of an ideal gas from initial volume V (initial) = 20.0 L to final volume V(final) = 40.0 L if n = 2.00 mol and T = 300 K.
Show the difference W(non-ideal) – W(ideal). If all your calculations are done correctly, this result shows you the effect of attractive interaction between gas particles on the work done by the system.
The work done of reversible, isothermal expansion is given by:
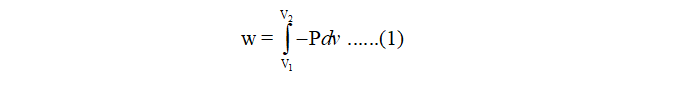
where P is the internal pressure of gas and dv is the change in volume
Step by step
Solved in 4 steps with 6 images









