Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:This image displays a selection ranking task focused on determining the strongest base among three given compounds. Each compound is depicted with its molecular structure.
1. **Compounds (from top to bottom):**
- The first compound shows a molecular structure with an ethyl group attached to an anion, likely indicating a base.
- The second compound features a ketone-like structure, with a simple anion attached.
- The third compound has a methyl group, again indicating a base with an anion.
2. **Ranking Section:**
- To the right, there is a vertical list meant for ranking the compounds. It is labeled as "Strongest base."
- There are three circular placeholders numbered 1, 2, and 3, where the compounds can be ranked from strongest to weakest base.
3. **Arrow:**
- An arrow in the middle points from the list of compounds toward the ranking section, indicating the action of ranking based on basicity.
This diagram is likely used to help students understand and compare the basic strength of different compounds by examining their structural features.

Transcribed Image Text:The image appears to be part of a ranking or comparison system, possibly for identifying the basicity of different chemical compounds. The section visible in the image is labeled as "Weakest base."
- The number "3" is prominently displayed within a large circle.
- Below this, the number "4" is enclosed in a smaller circle, with an arrow pointing downward.
- The molecular structure shown represents a carbonyl group attached to a three-carbon ring. The carbonyl group consists of a carbon double-bonded to an oxygen atom, which has two lone pairs of electrons drawn.
- This structure is likely illustrating a specific chemical compound in the context of its basicity.
The text "Weakest base" suggests that this compound is considered less basic compared to others in the system depicted in the full image.
The diagram helps visualize the relative basicity of the chemical compound shown, with the numeric labels indicating its position in a sequence or hierarchy.
Expert Solution
Step 1
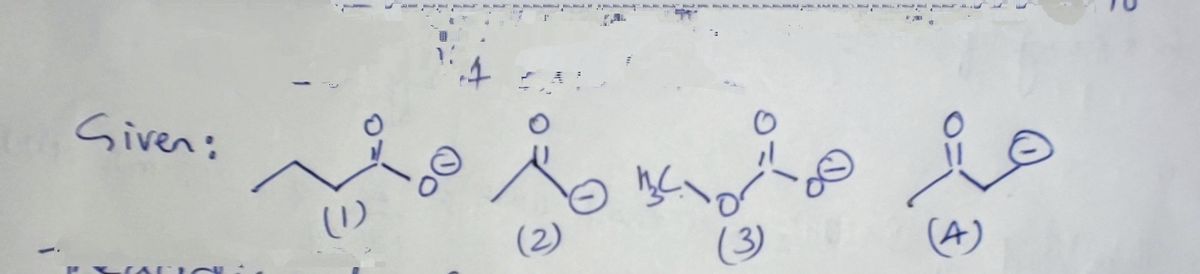
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY