Starting with the rate constant from your graphs and the NaOH concentrations, show haw you determined the order of the reaction with reapect to sodiom hydroxide.
Catalysis and Enzymatic Reactions
Catalysis is the kind of chemical reaction in which the rate (speed) of a reaction is enhanced by the catalyst which is not consumed during the process of reaction and afterward it is removed when the catalyst is not used to make up the impurity in the product. The enzymatic reaction is the reaction that is catalyzed via enzymes.
Lock And Key Model
The lock-and-key model is used to describe the catalytic enzyme activity, based on the interaction between enzyme and substrate. This model considers the lock as an enzyme and the key as a substrate to explain this model. The concept of how a unique distinct key only can have the access to open a particular lock resembles how the specific substrate can only fit into the particular active site of the enzyme. This is significant in understanding the intermolecular interaction between proteins and plays a vital role in drug interaction.
![(The calculations are NOT allowed on the concept review, so make sure you fully
understand them. Keep in lab manual to use to study for the lab practical.)
You will need to perform some of these, and similar, calculations on your concept review.
Graphs must be included as part of your results section.
Diluted concentration of reactants
Since you used equal amounts of both sodium hydroxide and crystal violet, you must divide the
original concentrations of each reactant by 2 to account for dilution when mixing the two solutions
together.
Diluted concentration of 1.0x10-5M crystal violet
6.구0 m L
6.70 mL
Diluted concentration of 0.010M NaOH
0.7omL
Diluted concentration of 0.020M NAOH
Correcting for negative absorbance readings (If necessary!)
If you get any negative absorbance readings, you need to add the positive value of the largest
negative reading to each of your absorbances. This will allow you to take the logarithm of the
number without an error. For example, if your largest negative absorbance reading is -0.035, then
you must add +0.035 to each of your absorbance readings. By doing this, your last value will be
0.000 instead of a negative value.
absorbance values from our data into concentration.
First reading: 0.102 would now be 0.137
Last reading: -0.035 would now be 0.000
Molar absorptivity of crystal violet, ɛ (Abs/M)
The absorbance is linearly related to the concentration of crystal violet based on Beer's Law:
A=ebc
A is the absorbance of the diluted crystal violet, b is the pathlength of the light (1.00cm) and c is
diluted concentration of the crystal violet. ɛ is the molar absorptivity and is a conversion factor to
convert the absorbance values from our data into concentration in molarity. To find the molar
absorptivity, divide the absorbance of the diluted crystal violet by the concentration of the diluted
crystal violet. The pathlength, b, cancels out since it has a value of 1cm. This value will be the same
for both concentrations of NaOH, as the absorbance of NaOH is essentially zero. Note; this
conversion factor will be a very large number as the concentration of the diluted crystal violet
is very low.
E(Abs/M) = (abs of diluted CV solution ) / [diluted CV]
0.423
O.6604
:3
0.70
41](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd4d956b8-1363-4a6a-8e95-0f238c52dd5a%2F54d5a1a2-55a0-438a-a3b2-6bb0c4e23c8b%2F5gdkyt_processed.jpeg&w=3840&q=75)
![Change
Colors
X v fx
D
Concentration (M) In[CV
orbance
1/[CV]
K
M
0 order 0.010 NAOH
0.43
0.71192053 -0.339789 1.4046512
0.416
0.688741722 -0.3728889 1.4519231
0.7
0.407
0.67384106
-0.394761 2.4840295
0.65397351 -0.4246884 1.5291139
0.6
0.395
y0.016 0.5946
0.
0.385
0.637417219 0.4503309 1.5688312
0.4
0.373
0.617549669 -0.4819958 1.6193029
Second order NAOH
0.363
0.600993377 -0.5091714 1.6639118
03
0.353
0.584437086 -0.5371061 1.7110482
0.2
0.343
0.567880795 -0.5658438 1.7609329
0.1
0.334
0.552980132 -0.5924332 1.8083832
0.51986755 -0.6541812 1.9235669
0.314
10
15
20
25
10
35
0.297
0.491721854 -0.7098421
2.03367
0.279
0.46192053 -0.7723624 2.1648746
9
0.263
0.435430464 -0.8314202 2.2965779
0.410596026 -0.8901455 2.4354839
First order 0.010 NAOH
10
0.248
11
0.235
0.389072848 -0.9439887 2.5702128
0.36589404 -1.0054115 2.7330317
10
15
20
25
30
35
12
0.221
13
0.21
0.347682119 -1.0564667 2.8761905
0.32615894 -1.1203705 3.0659898
y0.0-0299
10
15
20
25
30
35
R0.9895
14
0.197
-1
15
0.186
0.30794702-1.1778275 3.2473118
16
0.177
0.293046358-1.2274245 3.4124294
-1.5
17
0.167
0.276490066 -1.2855804 3.616766S
18
0.158
0.261589404 -1.3409792 3.8227848
0.248344371-1.3929389 4.0266667
-2
19
0.15
20
0.142
0.235099338-1.4477471 4.2535211
2.5
21
0.135
0.223509934 -1.4982994 4.4740741
22
0.127
0.210264901-1.5593871 4.7559055
23
0.121
0.200331126-1.6077837 4.9917355
Series "In[CV]" Trendline 1 Equation
24
0.115
0.190397351-1.6586421 5.2521739
25
0.109
0.180463576-1.7122263 5.5412844
26
0.104
0.17218543-1.7591833 S.8076923
27
28
0.165562914 -1.798404
0.160596026-1.8288632 6.2268041
0.1
6.04
0.097
29
0.091
0.150662252-1.8927147 6.6373626
30
0.086
0.142384106 -1.9492269 7.0232558
44
Sheet1
Sheet2
Ready
FEB 1
23
étv J A O
Starting with
the
the rate constant from
your graphs and
NaOHt concentrations, Show haw you
determined the order of
the reaction
with
respect to sodiom hydroxide.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd4d956b8-1363-4a6a-8e95-0f238c52dd5a%2F54d5a1a2-55a0-438a-a3b2-6bb0c4e23c8b%2Fa99umm_processed.jpeg&w=3840&q=75)
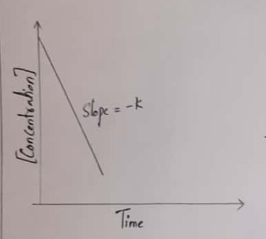
For a zeroth-order reaction, a plot of the concentration of any reactant versus time is a straight line with a slope of −k.
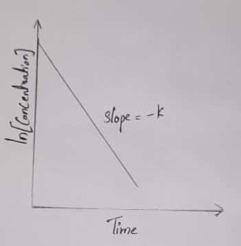
For a First-order reaction, a plot of the log[concentration] of any reactant versus time is a straight line with a slope of −k.
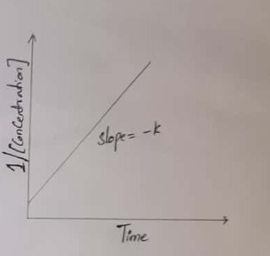
For a Second-order reaction, a plot of the log[concentration] of any reactant versus time is a straight line with a slope of −k.
Step by step
Solved in 3 steps with 4 images









