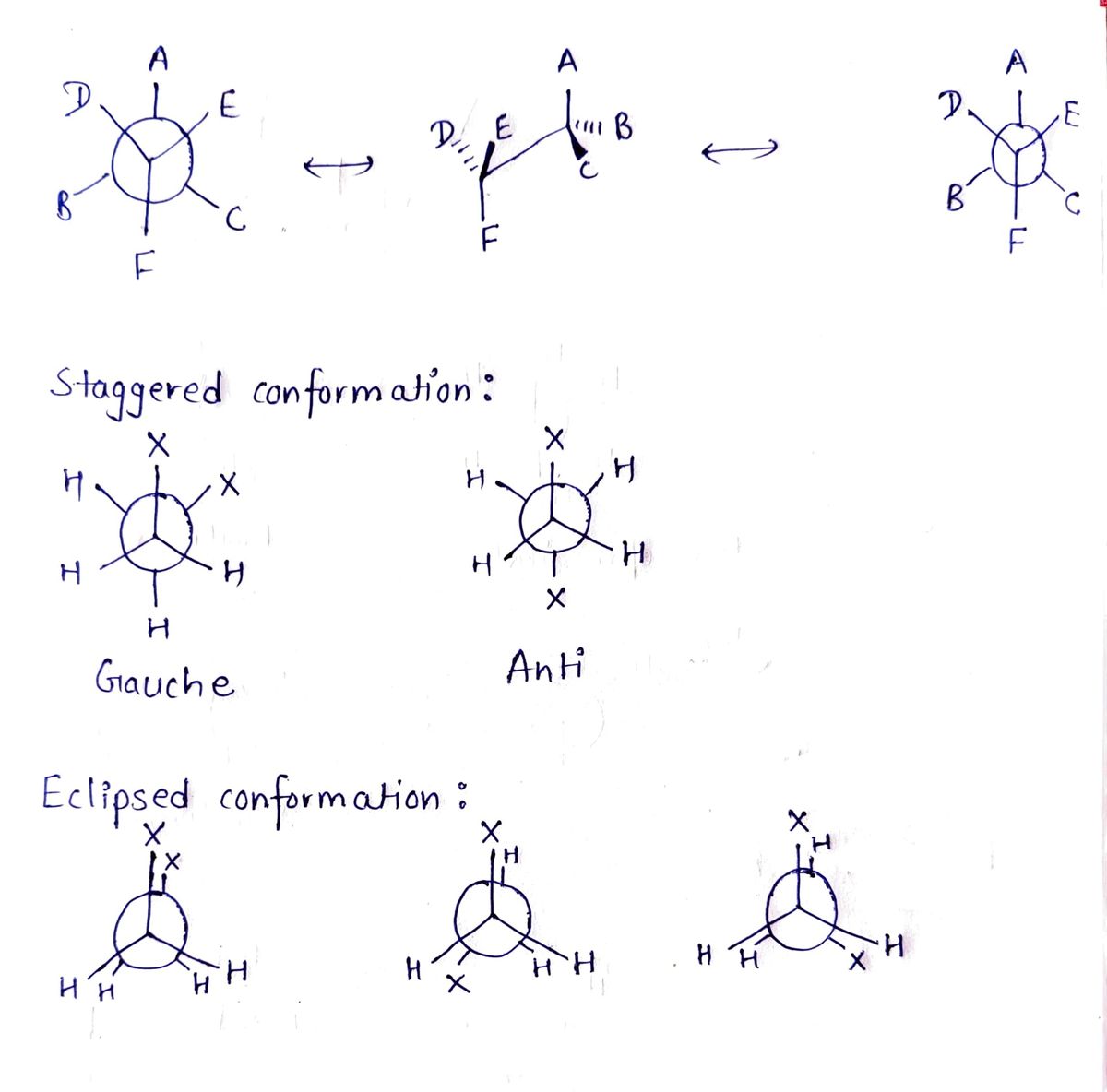
Starting from the Newman projection below, rotate only the back carbon to provide the structure in an anti staggered conformation. CH3 H C(CH3)3 Q H H H Drawing Version: 3.115.30 +4493 production Atoms, Bonds and Rings H CH₂CH₂ Charges CH(CH₂)z C(CH₂) CH3 CH3 Ph CH₂CH3 H 49 Undo Ⓡ Remove 17 Reset Ⓒ Done Submit Rotate
Starting from the Newman projection below, rotate only the back carbon to provide the structure in an anti staggered conformation. CH3 H C(CH3)3 Q H H H Drawing Version: 3.115.30 +4493 production Atoms, Bonds and Rings H CH₂CH₂ Charges CH(CH₂)z C(CH₂) CH3 CH3 Ph CH₂CH3 H 49 Undo Ⓡ Remove 17 Reset Ⓒ Done Submit Rotate
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Title: Understanding Newman Projections in Organic Chemistry**
**Scenario: Newman Projection Manipulation**
**Instructions:**
Starting from the Newman projection below, rotate only the back carbon to provide the structure in an anti-staggered conformation.
**Left Diagram:**
- The Newman projection features a molecule viewed along the carbon-carbon bond axis.
- The front carbon is depicted with three hydrogen atoms (H) and a central connection to a CH₃ group.
- There's also a C(CH₃)₃ group at the back carbon position, though explicit connections are not detailed.
**Right Diagram:**
- Shows a circle representing the rear carbon.
- Black lines extend from the center, indicating the spatial orientation of attached groups.
- Current orientation includes CH₃ and H groups.
- CH₃ is highlighted in red, suggesting it represents the group targeted for rotation to achieve the anti-staggered conformation.
**Tools and Options:**
- Buttons to manipulate atoms, bonds, and rings.
- Options to rotate, undo, reset, and confirm changes.
This interactive tool aids in visualizing and understanding the spatial arrangement of substituents in organic molecules using Newman projections.
Expert Solution
Step 1: Conformations
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY