段階的に解決し、 人工知能を使用せず、 優れた仕事を行います ご支援ありがとうございました SOLVE STEP BY STEP IN DIGITAL FORMAT DON'T USE AI | DON'T USE AI | DON'T USE AI | DON'T USE AI | 6. The depletion of ozone (O_3) in the stratosphere has been a topic of great concern among scientists in recent years. It is believed that ozone can react with nitric oxide (NO) that comes from aircraft emissions at high altitudes. The reaction is 03 + NO → O2 + NO2 If 0.740 g of Os reacts with 0.670 g of NO, how many grams of NO_2 will be produced? Which compound is the limiting reactant? Calculate the mass of the excess reactant that is recovered by finish the reaction.
Atmospheric Pollution
In the atmosphere, the existence of large quantities of undesirable substances that could cause several health issues to living organisms and humans is atmospheric pollution. Air pollution is otherwise known to be atmospheric pollution. The presence of undesirable materials would also destruct the natural environment such as a change in climate, degradation of habitat, or depletion of ozone. Air pollution is generated by the natural processes and activities of humans.
Smokestack Scrubbers
Once we believe in the environment and remember the pollution-producing facets of it, we will consider the smoke-stacks scrubber to be a number of the worst offenders. Although this is often legally correct, smoke-stack scrubbers often have a big function in terms of keeping ground-level air pollutants- safe to breathe and assisting within the management of emissions.

Details 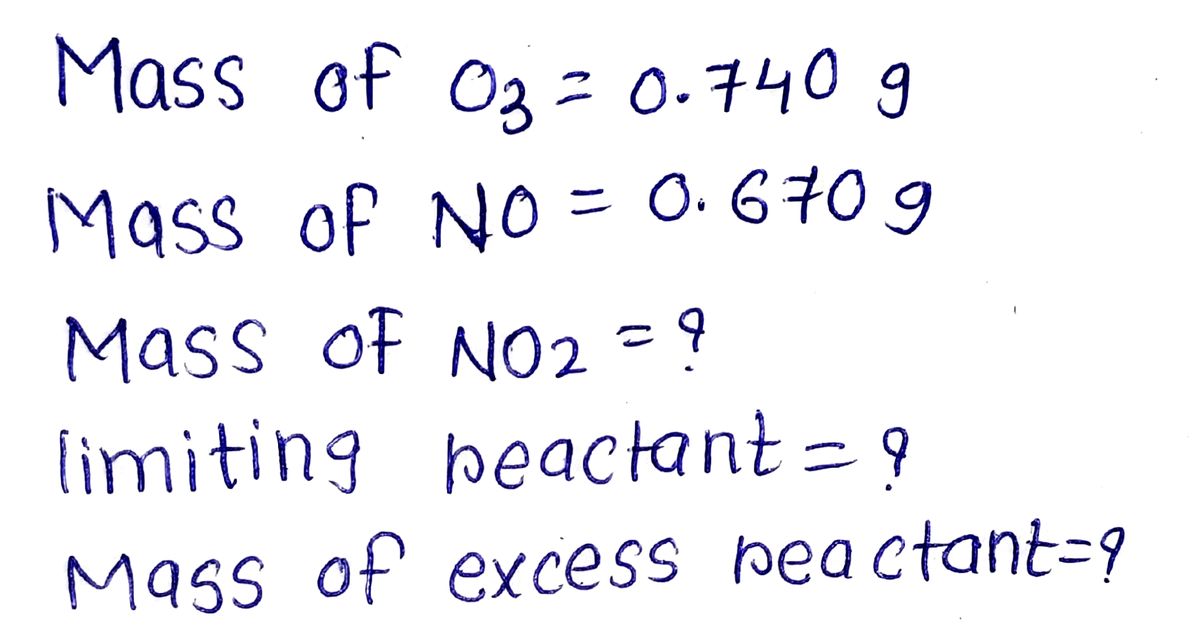
Step by step
Solved in 4 steps with 4 images









