SNAME and DRAW the STRUCTURE of ALL the alkenes which could undergo catalytic hydrogenation at 900°C to form methylcyclopentane. Circle the alkene with the HIGHEST stability and X the alkene with the HIGHEST heat of hydrogenation. Give reasons for your choice.
SNAME and DRAW the STRUCTURE of ALL the alkenes which could undergo catalytic hydrogenation at 900°C to form methylcyclopentane. Circle the alkene with the HIGHEST stability and X the alkene with the HIGHEST heat of hydrogenation. Give reasons for your choice.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Prompt:**
Name and draw the structure of all the alkenes which could undergo catalytic hydrogenation at 900°C to form methylcyclopentane. Circle the alkene with the highest stability and X the alkene with the highest heat of hydrogenation. Give reasons for your choice.
**Explanation for Educational Website:**
This prompt requires students to identify and illustrate all possible alkenes that can be hydrogenated to yield methylcyclopentane. The task involves three main components:
1. **Identification and Drawing:**
- Students need to recognize which structural alkenes are capable of transforming into methylcyclopentane through catalytic hydrogenation.
- Detailed structural drawings of these alkenes should be provided.
2. **Stability and Heat of Hydrogenation:**
- The alkene with the highest stability should be circled. Generally, more substituted alkenes tend to be more stable due to hyperconjugation and the inductive effect.
- The alkene with the highest heat of hydrogenation should be marked with an "X". Alkenes that are less stable have a higher heat of hydrogenation.
3. **Reasoning:**
- Students should include explanations for their choices, referencing concepts such as alkene stability (based on substitution patterns) and exothermic hydrogenation reactions (linked to stability).
This exercise helps students comprehend key aspects of alkene chemistry, including structural analysis, stability factors, and the thermodynamics of hydrogenation reactions.
Expert Solution
Step 1
Four alkenes are possible which on catalytic hydrogenation gives methyl cyclopentane.
All the structures are shown in image.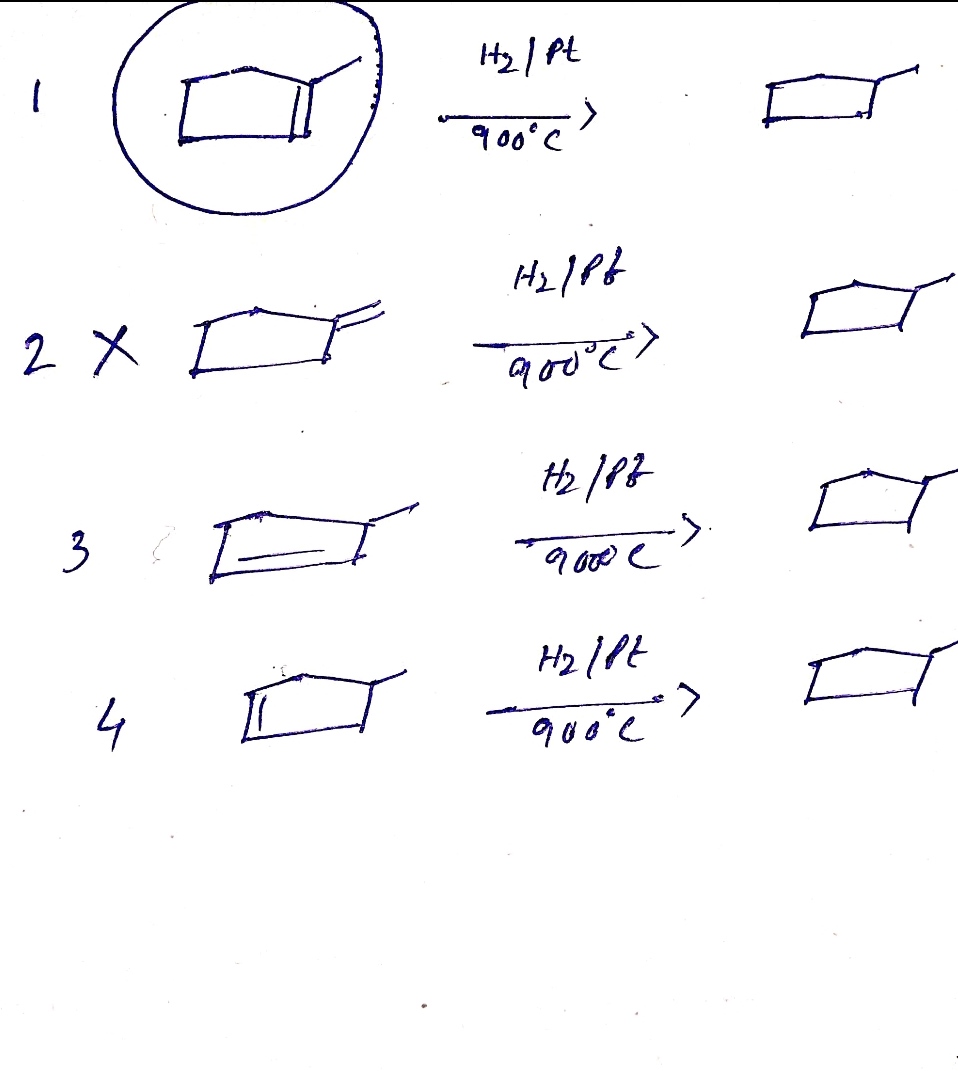
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY