Show reagents and experimental conditions to synthesize the following compound from 1-propanol
Q.) Methyloxirane
In primary alcohol, the -OH functional group is bonded to the carbon that has a nearby by the carbon atom. Example: 1-propanol. The removal of the -OH group from the primary alcohol produces the less stable primary carbocation. The presence of an oxygen as a ring constituent in a three-membered ring produces epoxide and it is also termed as oxirane. The alkene functional group is identified by the presence of a double bond. The reaction of an oxidizing agent with an alkene includes the oxygen to the alkene to yield a new compound.
Reaction:
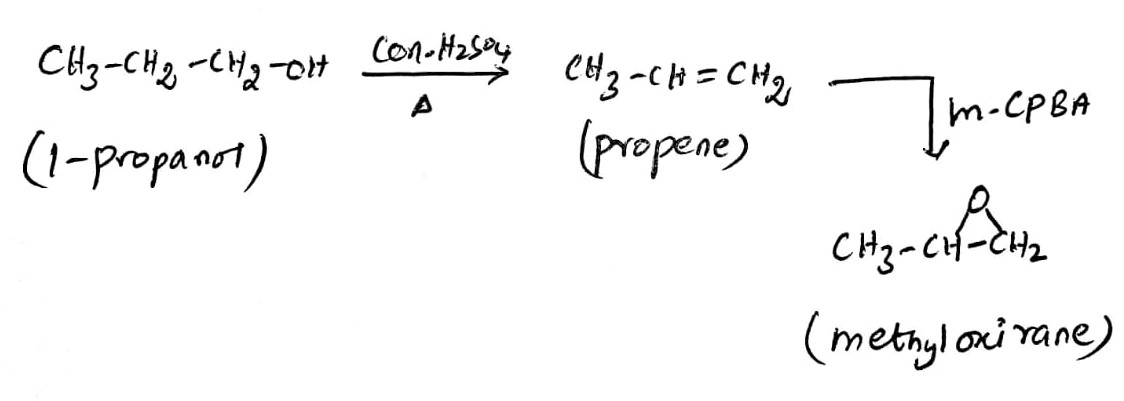
Explanation:
The reaction of 1-propanol with the concentrated sulfuric acid results in the formation of dehydration product such as propene along with the removal of a water molecule. The reaction of alkene with the epoxidation reagent such as meta chloroperbenzoic acid (m-CPBA) produces the methyl oxirane.
Step by step
Solved in 3 steps with 1 images









