Select all that apply for saturated vapor: It refers to a point on a T-v diagram on a specific isobaric (constant pressure) line. It is about to vaporize. It is about to condense. Any heat removed will cause some of the vapor to condense. It is still a 100% vapor.
Select all that apply for saturated vapor:
|
|
It refers to a point on a T-v diagram on a specific isobaric (constant pressure) line. |
|
|
It is about to vaporize. |
|
|
It is about to condense. |
|
|
Any heat removed will cause some of the vapor to condense. |
|
|
It is still a 100% vapor. |
The saturated vapor is a substance that exists completely as vapor at its saturation temperature. On a T-V phase diagram, it exists as a line shown below:
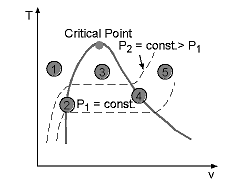
Point 1: Subcooled liquid region
Point 2: Saturated liquid line
Point 3: Saturated vapor-liquid region
Point 4: Saturated vapor line
Point 5: Superheated vapor region
Since it is totally a vapor at its saturated state, that is, on the verge of condensation, and any removal of heat will tend the vapor to condense.
Now,
- It refers to a point on a T-v diagram on a specific isobaric (constant pressure) line:
Does not apply as it is a line different from the isobaric line.
- It is about to vaporize:
Does not apply as it is already in vapor state and cannot vaporize further.
Step by step
Solved in 7 steps with 1 images









