Refer to the equation below to answer the questions that follow. P2 In P1 -Hvp T2 1 R T1 1* atım R = 0.0821 = 8.3144 mol * K mol K An organic compound made in the laboratory is distilled at reduced pressure in order to avoid decomposition. If the compound boils at 84°C at 15.8 mmHg pressure and has a normal boiling point of 296°C, what is the enthalpy of vaporization for this compound? O 31.0 kJ/mol O 33.2 kJ/mol O 3.78 kJ/mol O 0.306 kJ/mol
Refer to the equation below to answer the questions that follow. P2 In P1 -Hvp T2 1 R T1 1* atım R = 0.0821 = 8.3144 mol * K mol K An organic compound made in the laboratory is distilled at reduced pressure in order to avoid decomposition. If the compound boils at 84°C at 15.8 mmHg pressure and has a normal boiling point of 296°C, what is the enthalpy of vaporization for this compound? O 31.0 kJ/mol O 33.2 kJ/mol O 3.78 kJ/mol O 0.306 kJ/mol
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem ISP
Related questions
Question

Transcribed Image Text:Refer to the equation below to answer the questions that follow.
P2
In
P1
-Hup
T2
R
T1
1* atm
R = 0.0821
= 8.3144
mol * K
mol K
An organic compound made in the laboratory is distilled at reduced pressure in order to avoid decomposition. If the compound boils at 84°C at
15.8 mmHg pressure and has a normal boiling point of 296°C, what is the enthalpy of vaporization for this compound?
O 31.0 kJ/mol
O 33.2 kJ/mol
O 3.78 kJ/mol
O 0.306 kJ/mol
Expert Solution
Step 1
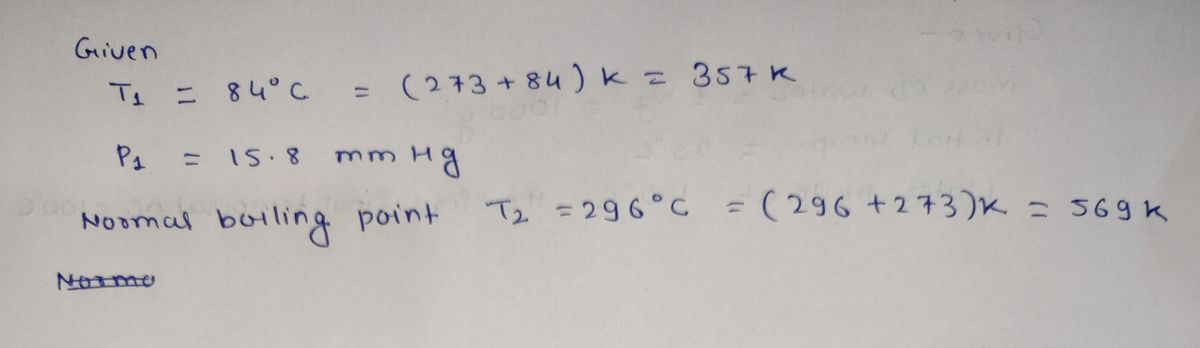
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning