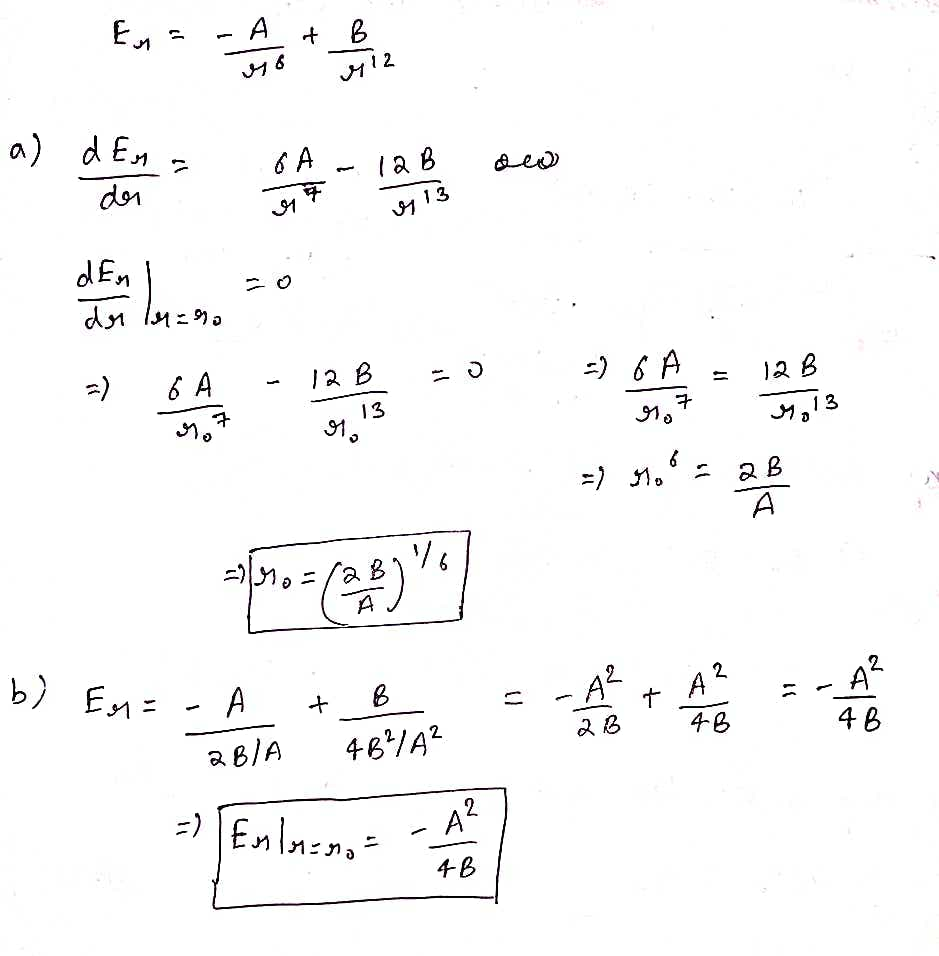
Question 5: Consider an interatomic potential function of the form A B Er= ===+ роб р12 a) Find the equilibrium bond length, ro, as a function of A and B. b) Derive the bond energy as a function of A and B. c) You are given that ro= 0.1 nm and Erl=-5 eV. Calculate values for A and B (remember to keep track of your units).
Question 5: Consider an interatomic potential function of the form A B Er= ===+ роб р12 a) Find the equilibrium bond length, ro, as a function of A and B. b) Derive the bond energy as a function of A and B. c) You are given that ro= 0.1 nm and Erl=-5 eV. Calculate values for A and B (remember to keep track of your units).
Related questions
Question

Transcribed Image Text:Question 5: Consider an interatomic potential function of the form
A B
12
Er=
76
a) Find the equilibrium bond length, ro, as a function of A and B.
b)
Derive the bond energy as a function of A and B.
c) You are given that ro= 0.1 nm and Erl=-5 eV. Calculate values for A and B (remember to keep
track of your units).
d) Take the second derivative of energy with respect to r and evaluate it at ro. This is effectively the
spring constant of the bond. Look up spring constants of some typical metal and ceramic materials
online. Where does your calculated value sit relative to these examples values?
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images
