Question attached

The given reaction is an example of the claisen condensation reaction which results in the formation of a beta-keto ester.
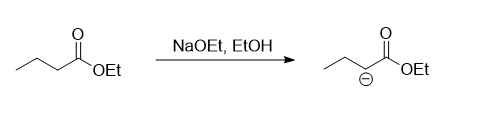
In the given reaction the two esters present are ethyl benzoate and ethyl butanoate. The ethyl benzoate does not consists of any hydrogen atom present at the alpha carbon of the carbonyl group, while there are two hydrogen atoms present on the alpha carbon of the carbonyl group in ethyl butanoate. So the first step of the reaction mechanism involves the abstraction of one of the hydrogen presents on the alpha carbon by the base NaOEt whic results in the formation of a carbanion.
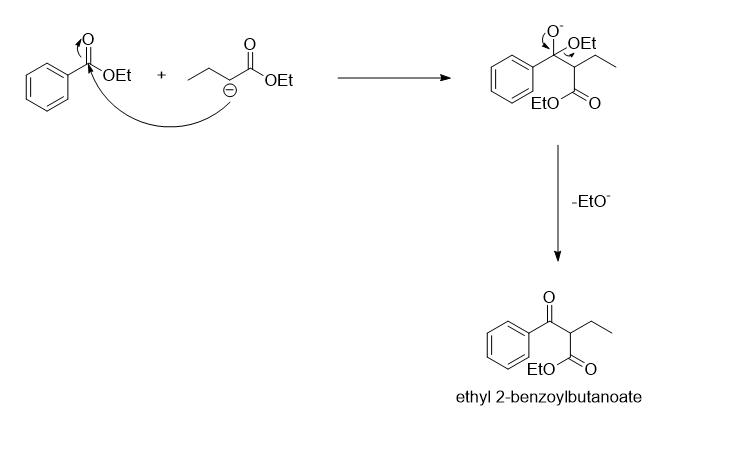
In the next step, the carbanion formed will act as a nucleophile and attacks on the carbonyl carbon of the ethyl benzoate followed by the removal of EtO- to form a beta-keto ester.
Step by step
Solved in 3 steps with 3 images









