Practice problems: H,0 H,SO4 1. Hg(0,C CF3)2, CH,ОН 2. NaBH4 1. ВН;/THF 2. ОН, Н,О2, Н,0 1. ВН+/THF 2. ОН, Н,О2, Н,0 1. ВН /THF 2. ОН, Н-0, Н,о
Can i get help with these problems

Given reactions:
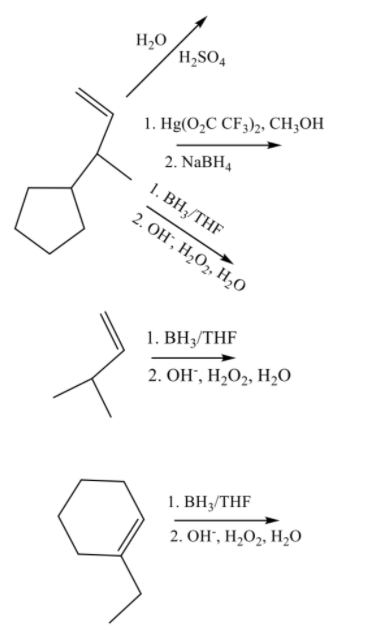
The addition of water molecule in the presence of sulfuric acid to an alkene leads to the formation of alcohol.
The product is formed according to Markovnikov rule which states that during addition of nucleophile to an asymmetric alkene the nucleophile gets attached to that carbon which contains a lesser number of hydrogen atoms.
The reaction of an alkene with mercury (II) trifluoroacetate in the presence of alcohol followed by the reaction with NaBH4 gives alkoxy compound.
The reaction of an alkene with diborane in the presence of an oxidizing agent to produce alcohol is termed as hydroboration oxidation. It follows anti-Markovnikov rule which states that during addition of nucleophile to an asymmetric alkene the nucleophile gets attached to that carbon which contains a more number of hydrogen atoms.
Step by step
Solved in 6 steps with 4 images









