Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Draw reaction mechanisms for the following:

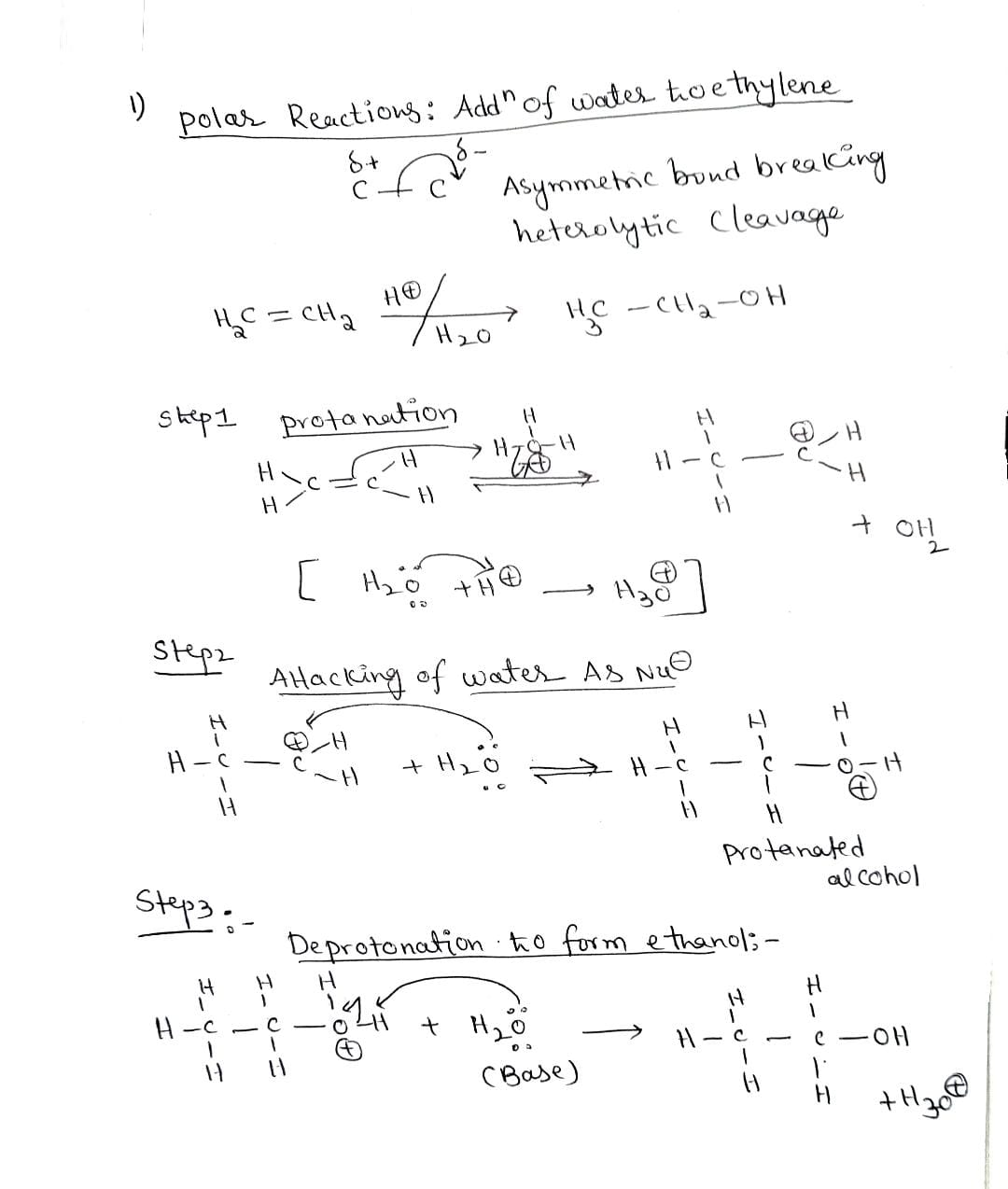
Transcribed Image Text:1. Polar Reactions: Addition of water to ethylene
This is an example of the polar reaction where asymmetric bond breaking and formation occur. When a covalent bond
is broken, the pair of electrons move to the same atom. When a covalent bond is formed, the electron pair comes from
the same atom.
Ethanol is produced when ethylene is heated with water and a strong acid catalyst, such as sulfuric acid. The reaction
mechanism can be visualized as proceeding in three steps. First, the alkene is protonated to produce a carbocation
intermediate. Then, water attacks the carbocation to form a protonated alcohol product. Finally, water acts as a base
yield back the acid catalyst and form the deprotonated alcohol product.
2. Radical Reactions: Free radical chlorination of methane
This is an example of a radical reaction where symmetric bond breaking and formation occur. When a covalent bond
is broken, the pair of electrons separate and move to different atoms. When a covalent bond is formed, the electron
pair comes from different atoms.
Like many radical reactions, chlorination of methane proceeds through three kinds of steps: initiation, propagation and
termination.
In the initiation step, UV breaks the relatively weak Cl–Cl bond to form Cl· radicals.
In the propagation step, the reactive chlorine radicals react with methane to abstract a hydrogen atom yielding HCl and
a methyl radical (CH3'). This methyl radical reacts with Cl2 in another propagation step to produce chloromethane and
a new chlorine radical, which is cycled back to the first propagation step. This then becomes a chain reaction of
repeating propagation steps.
In the termination step, two radicals collide to form a stable molecule, in which all electrons are paired. When this
happens, the reaction cycle is terminated as no new radicals are formed.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY