Part A Determine the empirical and molecular formulas of each of the following substances. Caffeine, a stimulant found in coffee, contains 49.5% C, 5.15% H, 28.9% N, and 16.5 % O by mass and has a molar mass of 195 g/mol. Express your answers as chemical formulas separated by a comma.
Part A Determine the empirical and molecular formulas of each of the following substances. Caffeine, a stimulant found in coffee, contains 49.5% C, 5.15% H, 28.9% N, and 16.5 % O by mass and has a molar mass of 195 g/mol. Express your answers as chemical formulas separated by a comma.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 6CR
Related questions
Question

Transcribed Image Text:Part A
Determine the empirical and molecular formulas of
each of the following substances.
Caffeine, a stimulant found in coffee,
contains 49.5% C, 5.15% H, 28.9% N, and 16.5
% O by mass and has a molar mass of 195
g/mol.
Express your answers as chemical formulas
separated by a comma.
Expert Solution
Step 1
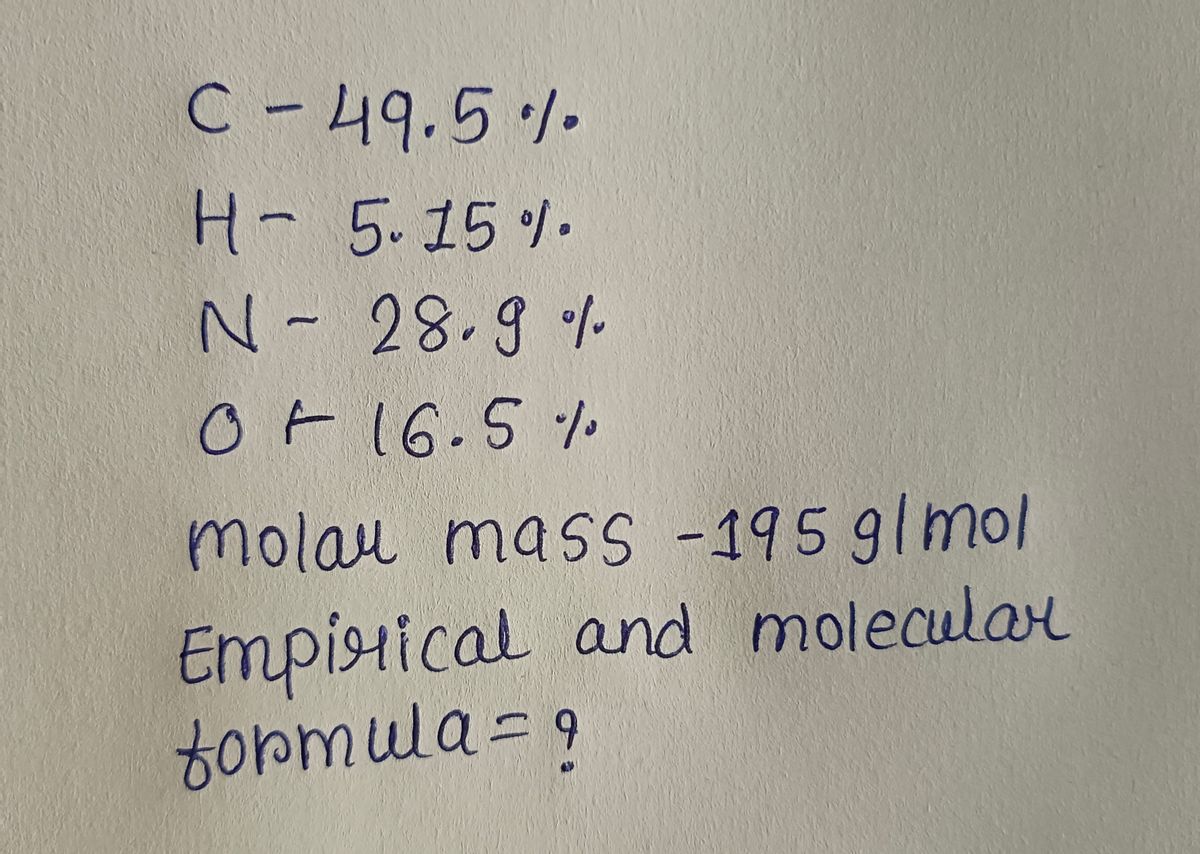
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning