Our talented team of bakery mixologists have designed the perfect formula for making our award winning Cuberry Cakes. The reaction ratio to mix our batter is 1 cup dry ingredients + 1 cup wet ingredients + 2 cups of berry mix3 cup batter Time(s) Wet ingredients (cups) Dry ingredients (cups) Berries (cups) Batter (cups) 10 10 9.75 10 9.5 1 0.75 9.75 9.5 5.4 1.5 9.5 9.25 9 9.0 8.5 8 13.8 9.25 2.25 3 3.75 4.5 26.3 9 43.1 8.75 7.5 8.75 8.5 8.25 64.3 8.5 89.8 120 5.25 6 8.25 6.5 8 6 Part 1: Based on the information in the above table, what is the average rate of the reaction (in cups/s) reported in terms formation of batter, from 13.8 s to 89.8 s? 乃5
Our talented team of bakery mixologists have designed the perfect formula for making our award winning Cuberry Cakes. The reaction ratio to mix our batter is 1 cup dry ingredients + 1 cup wet ingredients + 2 cups of berry mix3 cup batter Time(s) Wet ingredients (cups) Dry ingredients (cups) Berries (cups) Batter (cups) 10 10 9.75 10 9.5 1 0.75 9.75 9.5 5.4 1.5 9.5 9.25 9 9.0 8.5 8 13.8 9.25 2.25 3 3.75 4.5 26.3 9 43.1 8.75 7.5 8.75 8.5 8.25 64.3 8.5 89.8 120 5.25 6 8.25 6.5 8 6 Part 1: Based on the information in the above table, what is the average rate of the reaction (in cups/s) reported in terms formation of batter, from 13.8 s to 89.8 s? 乃5
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
Just part 1:
![Not Secure – loncapa5.chem.binghamton.edu
+ 88
00
LON-CAPA Q14-18
b Homework Help and Textbook Solutions | bartleby
Our talented team of bakery mixologists have designed the perfect formula for making our award winning Cuberry Cakes. The reaction ratio to mix our batter is
1 cup dry ingredients + 1 cup wet ingredients + 2 cups of berry mix
3 cup batter
Time(s) Wet ingredients (cups) Dry ingredients (cups) Berries (cups) Batter (cups)
10
10
10
1
9.75
9.75
9.5
0.75
5.4
9.5
9.5
9.0
1.5
13.8
9.25
9.25
8.5
2.25
26.3
9
9
43.1
8.75
8.75
7.5
3.75
64.3
8.5
8.5
|7
4.5
89.8
8.25
8.25
6.5
5.25
120
Part 1: Based on the information in the above table, what is the average rate of the reaction (in cups/s) reported in terms formation of batter, from 13.8 s to 89.8 s?
Submit Answer
Tries 0/99
Part 2: If the batter is appearing at a rate of 0.635 cups/second, what is the relative rate of disappearence of berries?
Submit Answer
Tries 0/99
Given the following rate law, rate =
k[Wet Ingredients] [dry ingredients]'[berries]', answer the following questions
Part 3: What is the order of the dry ingredients?
Submit Answer
Tries 0/99
Part 4: What is the overall order?
Submit Answer
Tries 0/99
Part 5: What are the units of k? (NOTE: This part is graded based on your answer Part 4. If you update your answer to the previous part, you MUST resubmit this question)
cups*s-1
s-1](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F17c38911-02f2-4fcc-a1c8-8efffd0de66b%2F2f0c598e-980e-4540-8378-1e9bf97759ce%2Firde1te_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Not Secure – loncapa5.chem.binghamton.edu
+ 88
00
LON-CAPA Q14-18
b Homework Help and Textbook Solutions | bartleby
Our talented team of bakery mixologists have designed the perfect formula for making our award winning Cuberry Cakes. The reaction ratio to mix our batter is
1 cup dry ingredients + 1 cup wet ingredients + 2 cups of berry mix
3 cup batter
Time(s) Wet ingredients (cups) Dry ingredients (cups) Berries (cups) Batter (cups)
10
10
10
1
9.75
9.75
9.5
0.75
5.4
9.5
9.5
9.0
1.5
13.8
9.25
9.25
8.5
2.25
26.3
9
9
43.1
8.75
8.75
7.5
3.75
64.3
8.5
8.5
|7
4.5
89.8
8.25
8.25
6.5
5.25
120
Part 1: Based on the information in the above table, what is the average rate of the reaction (in cups/s) reported in terms formation of batter, from 13.8 s to 89.8 s?
Submit Answer
Tries 0/99
Part 2: If the batter is appearing at a rate of 0.635 cups/second, what is the relative rate of disappearence of berries?
Submit Answer
Tries 0/99
Given the following rate law, rate =
k[Wet Ingredients] [dry ingredients]'[berries]', answer the following questions
Part 3: What is the order of the dry ingredients?
Submit Answer
Tries 0/99
Part 4: What is the overall order?
Submit Answer
Tries 0/99
Part 5: What are the units of k? (NOTE: This part is graded based on your answer Part 4. If you update your answer to the previous part, you MUST resubmit this question)
cups*s-1
s-1
Expert Solution
Step 1
Part 1
The average rate of reaction is calculated by dividing the change in concentration of the reactant or the change in concentration of the product over a specific time duration by the time interval.
Based on the above given information the average rate of reaction will be calculated as: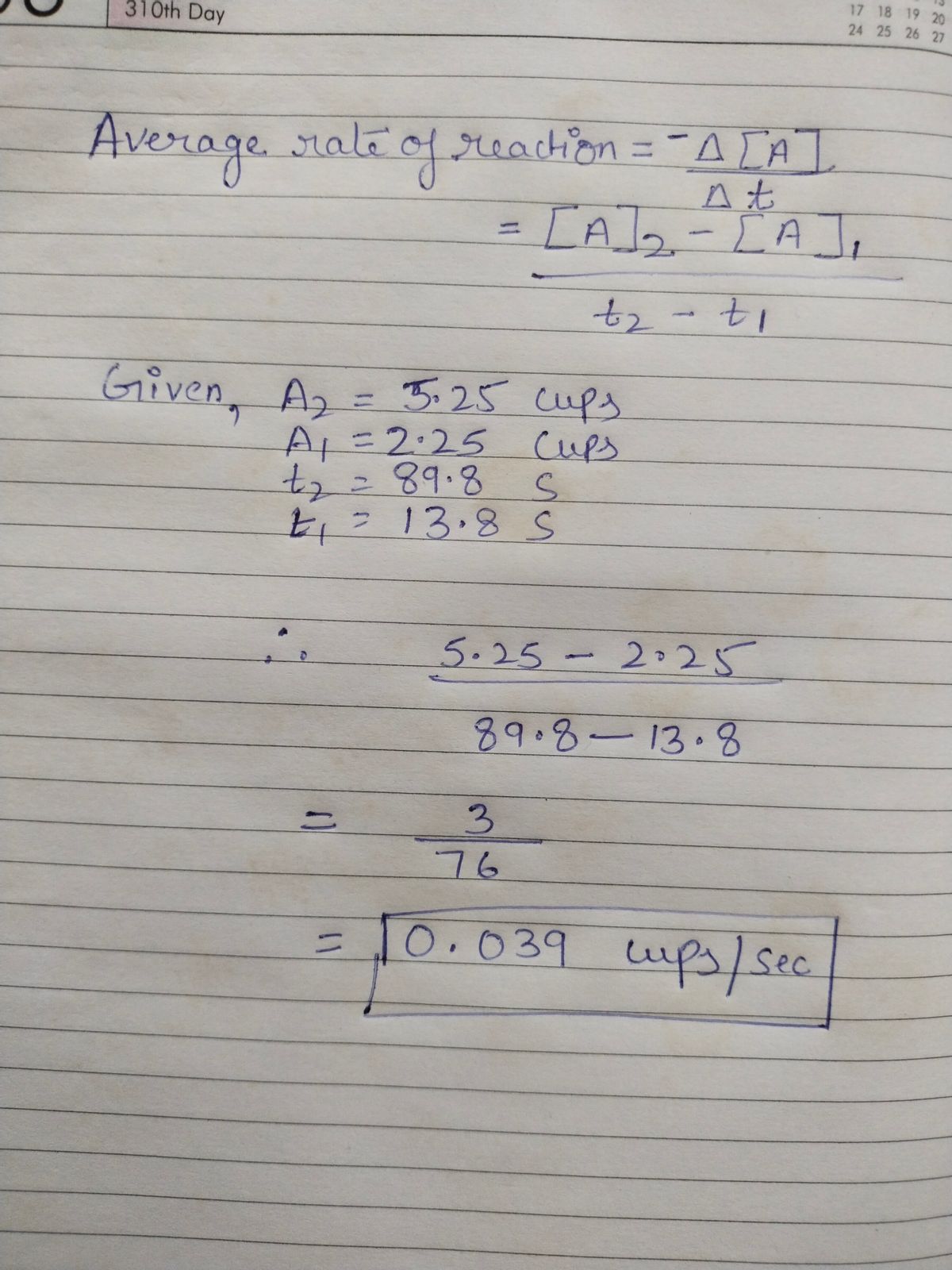
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON