ОН О OH ОН О H. B For the "crossed" aldol reaction that lead to product C, did the kind of base used make any difference to the reaction outcome? If so, what happened?
ОН О OH ОН О H. B For the "crossed" aldol reaction that lead to product C, did the kind of base used make any difference to the reaction outcome? If so, what happened?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Crossed Aldol Reaction Mechanism and Base Influence**
In the image, three chemical structures labeled A, B, and C are presented, relevant to a crossed aldol reaction. The compounds are:
- **Compound A:** A four-carbon chain with a terminal hydroxyl (OH) group and a carbonyl group (C=O), typical in aldol reactions.
- **Compound B:** A structure with a phenyl group attached, featuring a ketone group (C=O).
- **Compound C:** The final product, a combination of both structures, incorporating both the phenyl group and the carbonyl present in A and B, with an additional hydroxyl group suggesting a successful aldol condensation.
**Question:**
"For the 'crossed' aldol reaction that led to product C, did the kind of base used make any difference to the reaction outcome? If so, what happened?"
**Explanation:**
This question poses an inquiry about the role of the base in influencing the outcome of the crossed aldol reaction, focusing on the synthesis of product C. Crossed aldol reactions involve the reaction between two different carbonyl compounds, and the choice of base can affect the reaction course and selectivity. The outcome may involve observing changes in product yield, selectivity, or the presence of side products based on the base used. Understanding this helps in tailoring reaction conditions for desired outcomes in synthetic chemistry.
Expert Solution
Step 1
The structure of product C is represented as follows:
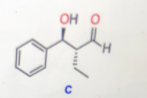
Step 2
The reaction in which two same or different carbonyl compounds containing alpha hydrogen are used with a base leads to removal of acidic hydrogen and forms an enolate ion which acts as a nucleophile for other carbonyl compound which is acting as an electrophile is termed as cross aldol reaction. If same carbonyl groups are used then there will be formation of 2 products but if different carbonyl groups are used then there will be formation of four different products.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY