Draw a complete, detailed mechanism for this reaction.

The product consists of a two phenyl groups that are joined via a carbon atoms. The structure is known as bisphenol A and it is a white solid compound. This reaction is an acid catalyzed condensation reaction.
The reactants given in the structure are acetone and toluene. The reaction takes place in the presence of a strong acid like sulfuric acid. Bisphenol A (BPA) is formed as the product. The reaction takes place in the presence of sulfonated styrene- divinylbenzene cation exchange resin. Water molecule is eliminated from the reaction.
The general equation is given as follows,
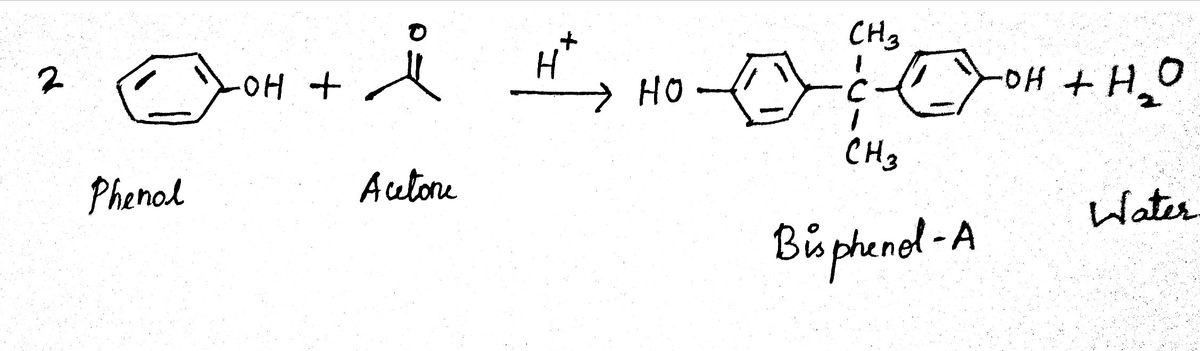
Two moles of phenol molecules are involved in the reaction. The resin used in the reaction is active only in its anhydrous form. Its action is controlled by water byproduct. In the reaction, phenol is used in excess. This enhances the yield of bisphenol A.
Step by step
Solved in 4 steps with 2 images









