North Carolina at Greensboro - CHEM 101 - Spring20 - WALSH > Activities and Due Dates > Ch 8 HW L Give Up? O Hint Check Answer D Assignment Score: 71.3% Resources < Question 15 of 18 > owing: A 1.569 g sample of a new organic material is combusted in a bomb calorimeter. The temperature of the calorimeter and its contents increase from 23.97 °C to 30.72 °C. per gram The heat capacity (calorimeter constant) of the calorimeter is 42.31 kJ/ °C, what is the heat of combustion of ct the material? ress kJ/g heat of combustion: % about us privacy policy terms of use contact us help careers scms/course/view.php?id%3D113397 3141593 36 MAR P. 29 MacBook Pro DII F5 F6 F8 F9 F10 F11 F12 F3 F4 8. т Y н J K в SPARTANS command option Kス %24 C. D. ప 0
North Carolina at Greensboro - CHEM 101 - Spring20 - WALSH > Activities and Due Dates > Ch 8 HW L Give Up? O Hint Check Answer D Assignment Score: 71.3% Resources < Question 15 of 18 > owing: A 1.569 g sample of a new organic material is combusted in a bomb calorimeter. The temperature of the calorimeter and its contents increase from 23.97 °C to 30.72 °C. per gram The heat capacity (calorimeter constant) of the calorimeter is 42.31 kJ/ °C, what is the heat of combustion of ct the material? ress kJ/g heat of combustion: % about us privacy policy terms of use contact us help careers scms/course/view.php?id%3D113397 3141593 36 MAR P. 29 MacBook Pro DII F5 F6 F8 F9 F10 F11 F12 F3 F4 8. т Y н J K в SPARTANS command option Kス %24 C. D. ప 0
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:North Carolina at Greensboro - CHEM 101 - Spring20 - WALSH > Activities and Due Dates > Ch 8 HW
L Give Up?
O Hint
Check Answer
D Assignment Score:
71.3%
Resources
< Question 15 of 18 >
owing:
A 1.569 g sample of a new organic material is combusted in a bomb calorimeter. The temperature of the calorimeter and its
contents increase from 23.97 °C to 30.72 °C.
per gram
The heat capacity (calorimeter constant) of the calorimeter is 42.31 kJ/ °C, what is the heat of combustion of
ct
the material?
ress
kJ/g
heat of combustion:
%
about us
privacy policy
terms of use
contact us
help
careers
scms/course/view.php?id%3D113397
3141593
36
MAR
P.
29
MacBook Pro
DII
F5
F6
F8
F9
F10
F11
F12
F3
F4
8.
т
Y
н
J
K
в
SPARTANS
command
option
Kス
%24
C.
D.
ప 0
Expert Solution
Step 1
The weight of sample is 1.569 g.
The given temperature values are 23.97 ˚C and 30.72 ˚C.
The constant value for specific heat is 42.31 kJ/g ˚C.
Step 2
The heat transfer during a reaction is given by,
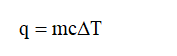
The temperature has increased, which means reaction is an exothermic reaction. Therefore,
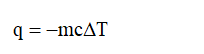
Step 3
Here, q is the heat energy, m is the mass of a substance, c is the specific heat, and ∆T is the change in temperature.
The change in temperature is,
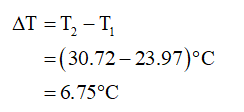
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY