Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
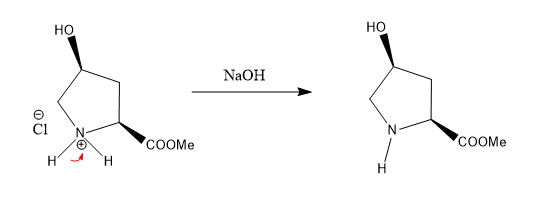
Show the mechanism for the following reaction.

Transcribed Image Text:The image illustrates a chemical reaction involving two reactants in a two-step synthesis process, resulting in an 80% yield of the product.
### Reactants:
1. **First Reactant**:
- Structure: A carbon backbone with an amide group and a tertiary butyl group. The amide group includes a proline derivative ("Pro") with an N-H bond.
- Functional Group: Alcohol (OH) group attached to the tertiary carbon.
2. **Second Reactant**:
- Structure: A ring with four carbon atoms and one nitrogen atom (pyrrolidinium chloride).
- Substituents:
- An OH group
- A positively charged nitrogen atom
- A methoxycarbonyl group (CO2Me)
- A chloride ion (Cl^−)
### Product:
- The product retains the proline derivative and incorporates the pyrrolidine ring from the second reactant. The nitrogeneous ring is integrated with the carbon structure from the first reactant.
- Functional Groups:
- An OH group attached to the ring
- A methoxycarbonyl group (CO2Me)
- The product maintains the amide bond.
### Reaction Details:
- **Process**: The transformation occurs over two steps.
- **Yield**: The overall reaction yield is 80%.
This reaction is an example of skilled organic synthesis, where a complex molecule is built from simpler structures. The process showcases the use of typical organic chemistry techniques and considerations such as maintaining functional groups and careful yield management.
Expert Solution
Introduction
The given reaction occurs in presence of base.
Step 1: Production of amine from ammonium salt in presence of base.
Step 2: Attack of amine on carbonyl carbon of carboxylic acid group and formation of amide bond.
Mechanism: Step 1
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY