NH2OH He CH3Cl CH4 c)Identify all types of intermolecular forces present d)Use dotted lines to illustrate the predominant intermolecular interaction between two 3-D drawn molecules of the same compound e)Rank the compounds in increasing order of boiling point using concepts from the Liquids and Solids chapter.
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
NH2OH He CH3Cl CH4
c)Identify all types of intermolecular forces present
d)Use dotted lines to illustrate the predominant intermolecular interaction between two 3-D drawn molecules of the same compound
e)Rank the compounds in increasing order of boiling point using concepts from the Liquids and Solids chapter.
The given molecules are:
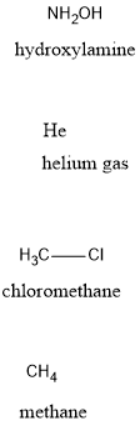
Regular molecular compounds have the following types of intermolecular forces:
1) Hydrogen bonding: Acts between the covalently bonded H-atom of one molecule and a highly electronegative atom(N,O,F) of another molecule in a substance.
For example:
HF ,NH3 and H2O etc. has intermolecular H-bonding.
2) Dipole-dipole interactions: They exist in polar covalent compounds.
Due to electronegativity differences between the bonded atoms, highly electronegative atom will attain partial positive charge and the other atom will attain partial positive charge.
So, two oppositely charged dipoles will be formed and the molecules are bind together by this dipole -dipole interactions.
3) London dispersion forces:
They exist in all the atoms and molecules.
These are the weakest intermolecular forces.
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images









