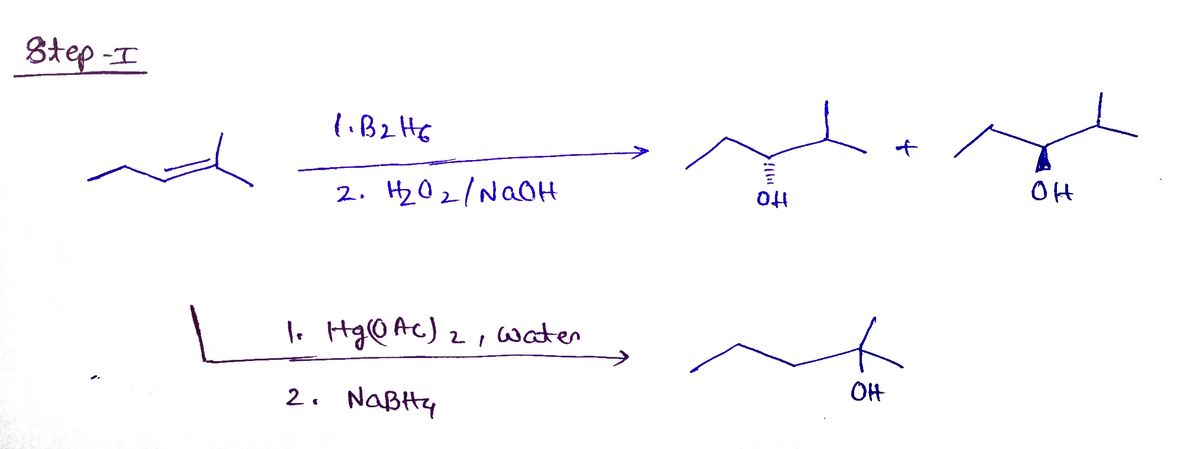
missing reagent to complete each reaction. Use a dash or wedge bond to indicate stereochemistry, where applicable. Ignore inorganic byproducts. Select I to Draw I 1. Hg(O Ac)2, water 2. NABH4. KMNO4. NaOH cold Select I to Draw 00
missing reagent to complete each reaction. Use a dash or wedge bond to indicate stereochemistry, where applicable. Ignore inorganic byproducts. Select I to Draw I 1. Hg(O Ac)2, water 2. NABH4. KMNO4. NaOH cold Select I to Draw 00
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Please help

Transcribed Image Text:### Organic Chemistry Reaction Pathways
This image provides an exercise in completing organic chemical reactions using the appropriate reagents. The task is to deduce and use the missing reagent to complete each reaction. Pay special attention to stereochemistry, using dash or wedge bonds where applicable. Inorganic byproducts are to be ignored.
#### Reaction Sequence Overview:
1. **First Reaction Series:**
- **Reactant:** A molecule with two hydroxyl groups.
- **Conditions:**
- Step 1: Use of mercury(II) acetate (Hg(OAc)₂) and water.
- Step 2: Reduction using sodium borohydride (NaBH₄).
2. **Intermediate Product:**
- The structure shown is a simplified cyclic molecule.
3. **Second Reaction Series:**
- The product from the first reaction acts as the reactant here.
- **Reagents:** Potassium permanganate (KMnO₄) and sodium hydroxide (NaOH) under cold conditions.
- **Expected Transformation:** Conversion to another organic product.
#### Diagram Explanation:
- **Boxes with Dotted Lines:** These represent places where the reagents or products need to be drawn or filled in.
- **Arrow Pathways:** Indicate the flow of the reaction from reactants to intermediates to products.
Use these guidelines to draw the missing reagents or products and complete the reaction pathway accurately.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY